 Found 77 hits with Last Name = 'thieme' and Initial = 'tm'
Found 77 hits with Last Name = 'thieme' and Initial = 'tm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337806
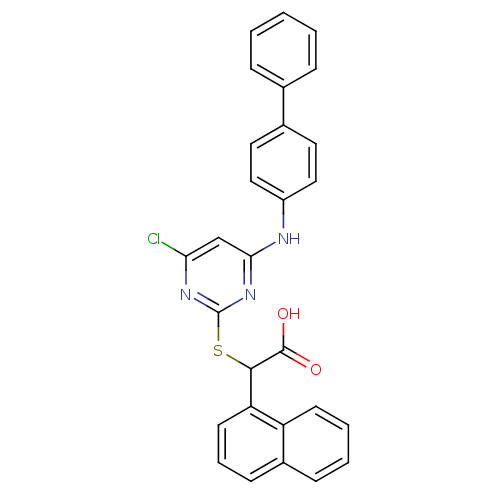
(2-(4-(biphenyl-4-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337808
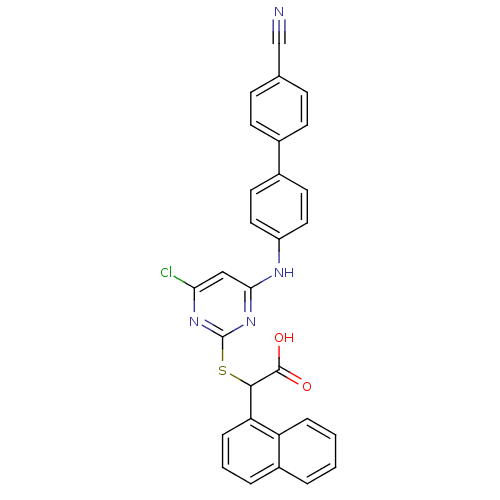
(2-(4-chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H19ClN4O2S/c30-25-16-26(32-22-14-12-20(13-15-22)19-10-8-18(17-31)9-11-19)34-29(33-25)37-27(28(35)36)24-7-3-5-21-4-1-2-6-23(21)24/h1-16,27H,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337813
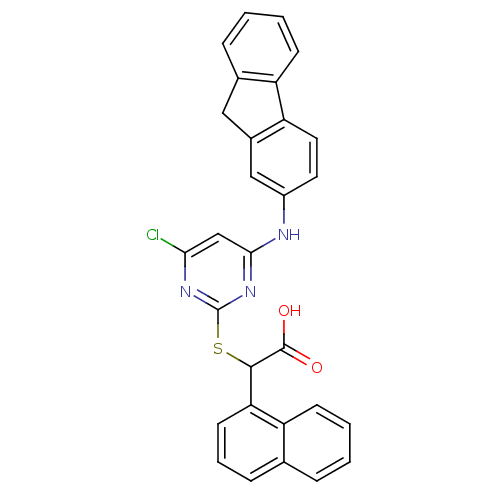
(2-(4-(9H-fluoren-2-ylamino)-6-chloropyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc-3c(Cc4ccccc-34)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H20ClN3O2S/c30-25-16-26(31-20-12-13-23-19(15-20)14-18-7-2-4-10-22(18)23)33-29(32-25)36-27(28(34)35)24-11-5-8-17-6-1-3-9-21(17)24/h1-13,15-16,27H,14H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337805
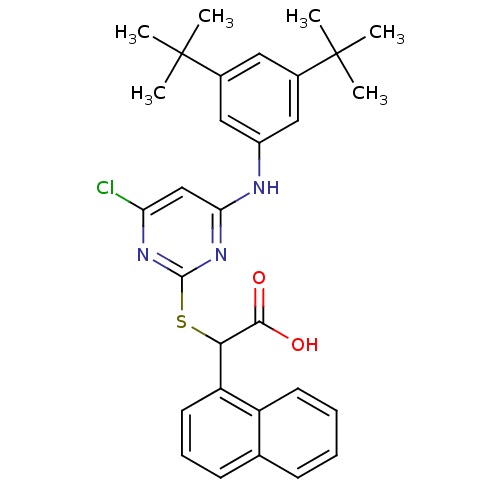
(2-(4-chloro-6-(3,5-di-tert-butylphenylamino)pyrimi...)Show SMILES CC(C)(C)c1cc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc(c1)C(C)(C)C Show InChI InChI=1S/C30H32ClN3O2S/c1-29(2,3)19-14-20(30(4,5)6)16-21(15-19)32-25-17-24(31)33-28(34-25)37-26(27(35)36)23-13-9-11-18-10-7-8-12-22(18)23/h7-17,26H,1-6H3,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315660
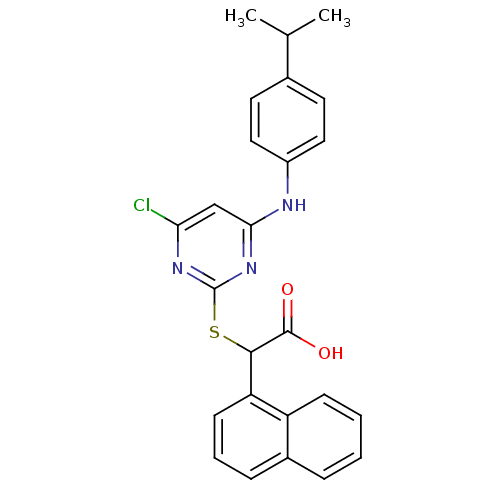
(2-(4-chloro-6-(4-isopropylphenylamino)pyrimidin-2-...)Show SMILES CC(C)c1ccc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc1 Show InChI InChI=1S/C25H22ClN3O2S/c1-15(2)16-10-12-18(13-11-16)27-22-14-21(26)28-25(29-22)32-23(24(30)31)20-9-5-7-17-6-3-4-8-19(17)20/h3-15,23H,1-2H3,(H,30,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337812
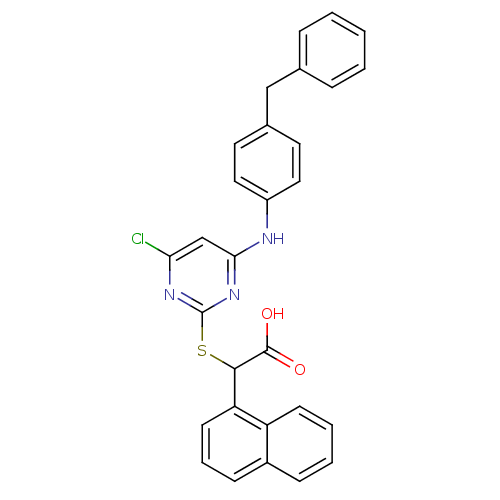
(2-(4-(4-benzylphenylamino)-6-chloropyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Cc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-18-26(31-22-15-13-20(14-16-22)17-19-7-2-1-3-8-19)33-29(32-25)36-27(28(34)35)24-12-6-10-21-9-4-5-11-23(21)24/h1-16,18,27H,17H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337809
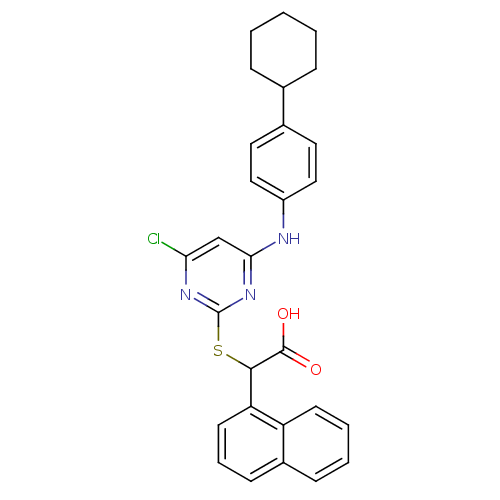
(2-(4-chloro-6-(4-cyclohexylphenylamino)pyrimidin-2...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)C2CCCCC2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H26ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h4-6,9-18,26H,1-3,7-8H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337810
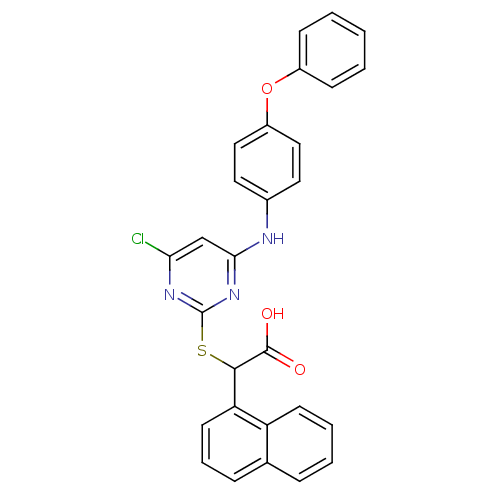
(2-(4-chloro-6-(4-phenoxyphenylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Oc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O3S/c29-24-17-25(30-19-13-15-21(16-14-19)35-20-9-2-1-3-10-20)32-28(31-24)36-26(27(33)34)23-12-6-8-18-7-4-5-11-22(18)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337807
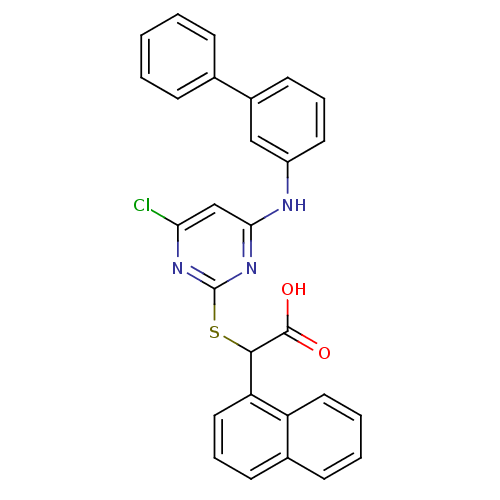
(2-(4-(biphenyl-3-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2cccc(c2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-13-6-12-20(16-21)18-8-2-1-3-9-18)32-28(31-24)35-26(27(33)34)23-15-7-11-19-10-4-5-14-22(19)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315657
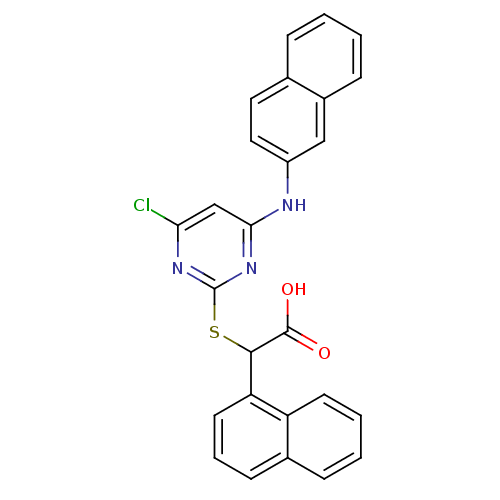
(2-(4-chloro-6-(naphthalen-2-ylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3ccccc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C26H18ClN3O2S/c27-22-15-23(28-19-13-12-16-6-1-2-8-18(16)14-19)30-26(29-22)33-24(25(31)32)21-11-5-9-17-7-3-4-10-20(17)21/h1-15,24H,(H,31,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315658
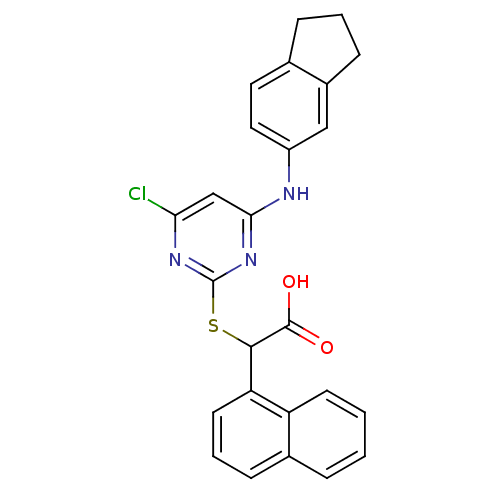
(2-(4-chloro-6-(2,3-dihydro-1H-inden-5-ylamino)pyri...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3CCCc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C25H20ClN3O2S/c26-21-14-22(27-18-12-11-15-6-3-8-17(15)13-18)29-25(28-21)32-23(24(30)31)20-10-4-7-16-5-1-2-9-19(16)20/h1-2,4-5,7,9-14,23H,3,6,8H2,(H,30,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337816
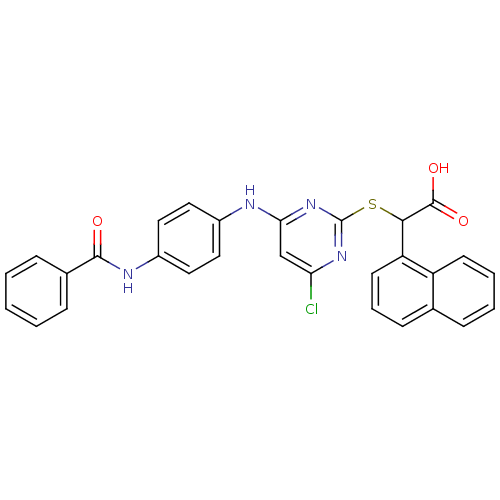
(2-(4-(4-benzamidophenylamino)-6-chloropyrimidin-2-...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(NC(=O)c3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H21ClN4O3S/c30-24-17-25(31-20-13-15-21(16-14-20)32-27(35)19-8-2-1-3-9-19)34-29(33-24)38-26(28(36)37)23-12-6-10-18-7-4-5-11-22(18)23/h1-17,26H,(H,32,35)(H,36,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315661
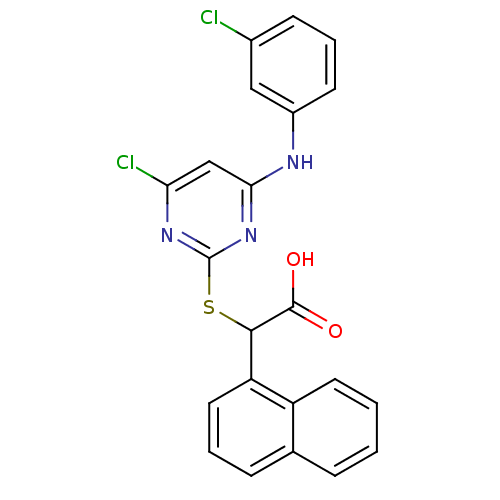
(2-(4-chloro-6-(3-chlorophenylamino)pyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2cccc(Cl)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C22H15Cl2N3O2S/c23-14-7-4-8-15(11-14)25-19-12-18(24)26-22(27-19)30-20(21(28)29)17-10-3-6-13-5-1-2-9-16(13)17/h1-12,20H,(H,28,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315659
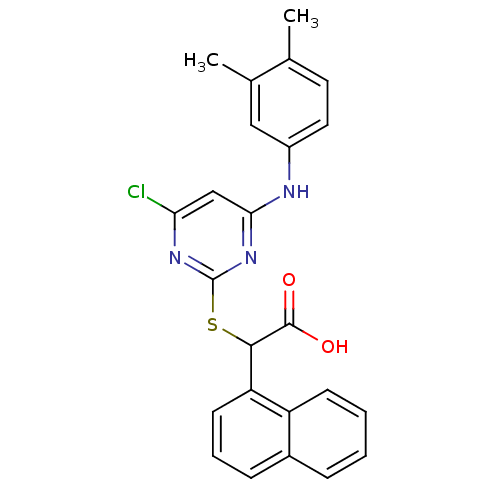
(2-(4-chloro-6-(3,4-dimethylphenylamino)pyrimidin-2...)Show SMILES Cc1ccc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc1C Show InChI InChI=1S/C24H20ClN3O2S/c1-14-10-11-17(12-15(14)2)26-21-13-20(25)27-24(28-21)31-22(23(29)30)19-9-5-7-16-6-3-4-8-18(16)19/h3-13,22H,1-2H3,(H,29,30)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337811
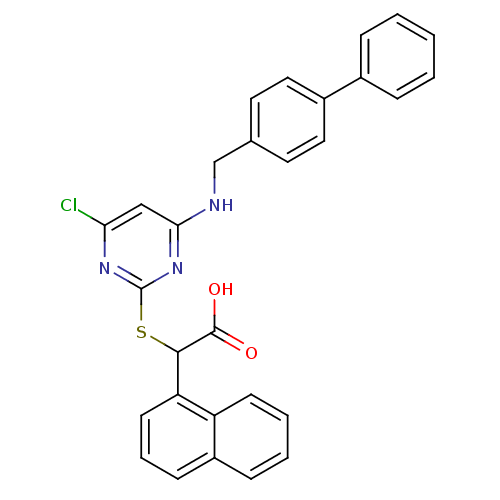
(2-(4-(biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-17-26(31-18-19-13-15-21(16-14-19)20-7-2-1-3-8-20)33-29(32-25)36-27(28(34)35)24-12-6-10-22-9-4-5-11-23(22)24/h1-17,27H,18H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443967
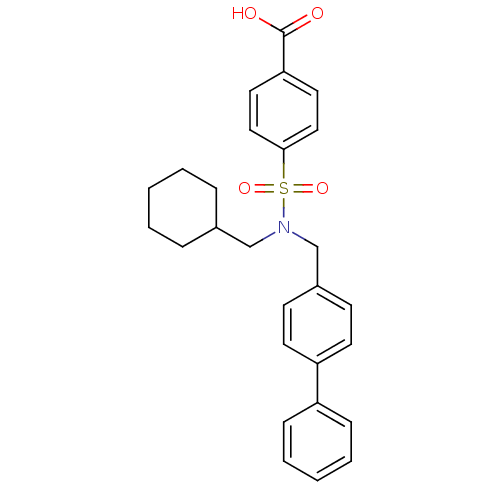
(CHEMBL3092283)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)N(CC1CCCCC1)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H29NO4S/c29-27(30)25-15-17-26(18-16-25)33(31,32)28(19-21-7-3-1-4-8-21)20-22-11-13-24(14-12-22)23-9-5-2-6-10-23/h2,5-6,9-18,21H,1,3-4,7-8,19-20H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human HeLa cells using PGH2 as substrate assessed as inhibition of IL-1beta/TNFalpha-stimulated PGE2 production preincubated... |
Bioorg Med Chem 21: 7874-83 (2013)
Article DOI: 10.1016/j.bmc.2013.10.006
BindingDB Entry DOI: 10.7270/Q2736SCJ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315662

(2-(4-chloro-6-(4-chlorophenylamino)pyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Cl)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C22H15Cl2N3O2S/c23-14-8-10-15(11-9-14)25-19-12-18(24)26-22(27-19)30-20(21(28)29)17-7-3-5-13-4-1-2-6-16(13)17/h1-12,20H,(H,28,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337811

(2-(4-(biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-17-26(31-18-19-13-15-21(16-14-19)20-7-2-1-3-8-20)33-29(32-25)36-27(28(34)35)24-12-6-10-22-9-4-5-11-23(22)24/h1-17,27H,18H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337805

(2-(4-chloro-6-(3,5-di-tert-butylphenylamino)pyrimi...)Show SMILES CC(C)(C)c1cc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc(c1)C(C)(C)C Show InChI InChI=1S/C30H32ClN3O2S/c1-29(2,3)19-14-20(30(4,5)6)16-21(15-19)32-25-17-24(31)33-28(34-25)37-26(27(35)36)23-13-9-11-18-10-7-8-12-22(18)23/h7-17,26H,1-6H3,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337810

(2-(4-chloro-6-(4-phenoxyphenylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Oc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O3S/c29-24-17-25(30-19-13-15-21(16-14-19)35-20-9-2-1-3-10-20)32-28(31-24)36-26(27(33)34)23-12-6-8-18-7-4-5-11-22(18)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337806

(2-(4-(biphenyl-4-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443968
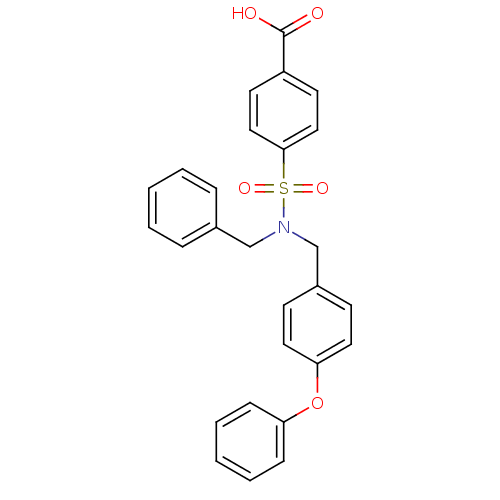
(CHEMBL3092271)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H23NO5S/c29-27(30)23-13-17-26(18-14-23)34(31,32)28(19-21-7-3-1-4-8-21)20-22-11-15-25(16-12-22)33-24-9-5-2-6-10-24/h1-18H,19-20H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human HeLa cells using PGH2 as substrate assessed as inhibition of IL-1beta/TNFalpha-stimulated PGE2 production preincubated... |
Bioorg Med Chem 21: 7874-83 (2013)
Article DOI: 10.1016/j.bmc.2013.10.006
BindingDB Entry DOI: 10.7270/Q2736SCJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337809

(2-(4-chloro-6-(4-cyclohexylphenylamino)pyrimidin-2...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)C2CCCCC2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H26ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h4-6,9-18,26H,1-3,7-8H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337812

(2-(4-(4-benzylphenylamino)-6-chloropyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Cc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-18-26(31-22-15-13-20(14-16-22)17-19-7-2-1-3-8-19)33-29(32-25)36-27(28(34)35)24-12-6-10-21-9-4-5-11-23(21)24/h1-16,18,27H,17H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337813

(2-(4-(9H-fluoren-2-ylamino)-6-chloropyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc-3c(Cc4ccccc-34)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H20ClN3O2S/c30-25-16-26(31-20-12-13-23-19(15-20)14-18-7-2-4-10-22(18)23)33-29(32-25)36-27(28(34)35)24-11-5-8-17-6-1-3-9-21(17)24/h1-13,15-16,27H,14H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337813

(2-(4-(9H-fluoren-2-ylamino)-6-chloropyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc-3c(Cc4ccccc-34)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H20ClN3O2S/c30-25-16-26(31-20-12-13-23-19(15-20)14-18-7-2-4-10-22(18)23)33-29(32-25)36-27(28(34)35)24-11-5-8-17-6-1-3-9-21(17)24/h1-13,15-16,27H,14H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337808

(2-(4-chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H19ClN4O2S/c30-25-16-26(32-22-14-12-20(13-15-22)19-10-8-18(17-31)9-11-19)34-29(33-25)37-27(28(35)36)24-7-3-5-21-4-1-2-6-23(21)24/h1-16,27H,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50443969
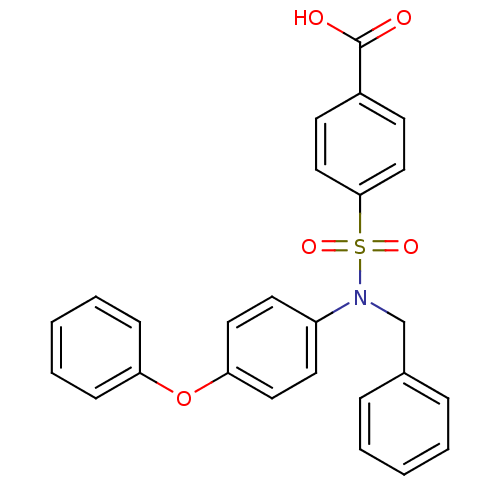
(CHEMBL3092270)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H21NO5S/c28-26(29)21-11-17-25(18-12-21)33(30,31)27(19-20-7-3-1-4-8-20)22-13-15-24(16-14-22)32-23-9-5-2-6-10-23/h1-18H,19H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human HeLa cells using PGH2 as substrate assessed as inhibition of IL-1beta/TNFalpha-stimulated PGE2 production preincubated... |
Bioorg Med Chem 21: 7874-83 (2013)
Article DOI: 10.1016/j.bmc.2013.10.006
BindingDB Entry DOI: 10.7270/Q2736SCJ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315666
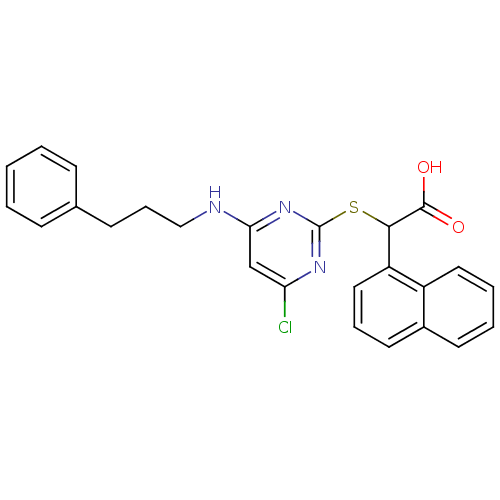
(2-(4-chloro-6-(3-phenylpropylamino)pyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(NCCCc2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C25H22ClN3O2S/c26-21-16-22(27-15-7-10-17-8-2-1-3-9-17)29-25(28-21)32-23(24(30)31)20-14-6-12-18-11-4-5-13-19(18)20/h1-6,8-9,11-14,16,23H,7,10,15H2,(H,30,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337805

(2-(4-chloro-6-(3,5-di-tert-butylphenylamino)pyrimi...)Show SMILES CC(C)(C)c1cc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc(c1)C(C)(C)C Show InChI InChI=1S/C30H32ClN3O2S/c1-29(2,3)19-14-20(30(4,5)6)16-21(15-19)32-25-17-24(31)33-28(34-25)37-26(27(35)36)23-13-9-11-18-10-7-8-12-22(18)23/h7-17,26H,1-6H3,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337807

(2-(4-(biphenyl-3-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2cccc(c2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-13-6-12-20(16-21)18-8-2-1-3-9-18)32-28(31-24)35-26(27(33)34)23-15-7-11-19-10-4-5-14-22(19)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50315657

(2-(4-chloro-6-(naphthalen-2-ylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3ccccc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C26H18ClN3O2S/c27-22-15-23(28-19-13-12-16-6-1-2-8-18(16)14-19)30-26(29-22)33-24(25(31)32)21-11-5-9-17-7-3-4-10-20(17)21/h1-15,24H,(H,31,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337809

(2-(4-chloro-6-(4-cyclohexylphenylamino)pyrimidin-2...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)C2CCCCC2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H26ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h4-6,9-18,26H,1-3,7-8H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337812

(2-(4-(4-benzylphenylamino)-6-chloropyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Cc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-18-26(31-22-15-13-20(14-16-22)17-19-7-2-1-3-8-19)33-29(32-25)36-27(28(34)35)24-12-6-10-21-9-4-5-11-23(21)24/h1-16,18,27H,17H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337806

(2-(4-(biphenyl-4-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337808

(2-(4-chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H19ClN4O2S/c30-25-16-26(32-22-14-12-20(13-15-22)19-10-8-18(17-31)9-11-19)34-29(33-25)37-27(28(35)36)24-7-3-5-21-4-1-2-6-23(21)24/h1-16,27H,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337814
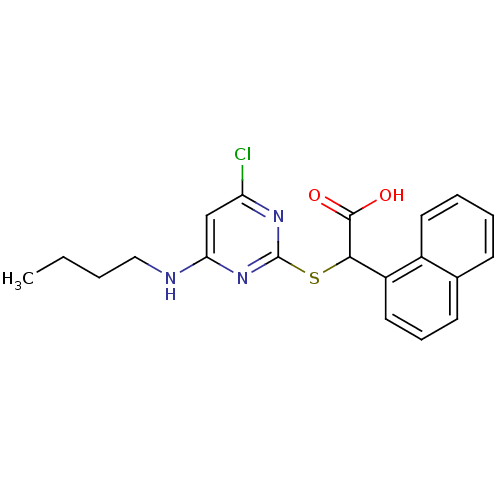
(2-(4-(butylamino)-6-chloropyrimidin-2-ylthio)-2-(n...)Show SMILES CCCCNc1cc(Cl)nc(SC(C(O)=O)c2cccc3ccccc23)n1 Show InChI InChI=1S/C20H20ClN3O2S/c1-2-3-11-22-17-12-16(21)23-20(24-17)27-18(19(25)26)15-10-6-8-13-7-4-5-9-14(13)15/h4-10,12,18H,2-3,11H2,1H3,(H,25,26)(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315664
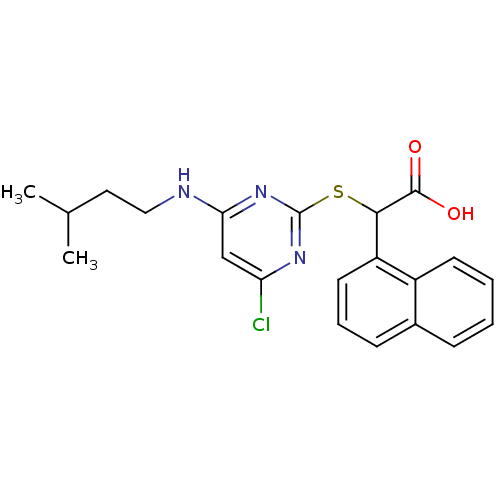
(2-(4-chloro-6-(isopentylamino)pyrimidin-2-ylthio)-...)Show SMILES CC(C)CCNc1cc(Cl)nc(SC(C(O)=O)c2cccc3ccccc23)n1 Show InChI InChI=1S/C21H22ClN3O2S/c1-13(2)10-11-23-18-12-17(22)24-21(25-18)28-19(20(26)27)16-9-5-7-14-6-3-4-8-15(14)16/h3-9,12-13,19H,10-11H2,1-2H3,(H,26,27)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315665
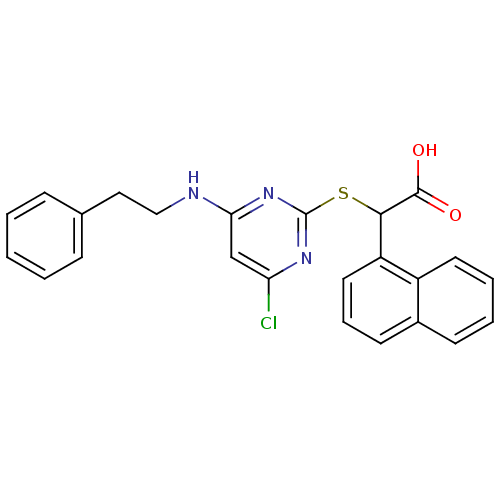
(2-(4-chloro-6-(phenethylamino)pyrimidin-2-ylthio)-...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(NCCc2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C24H20ClN3O2S/c25-20-15-21(26-14-13-16-7-2-1-3-8-16)28-24(27-20)31-22(23(29)30)19-12-6-10-17-9-4-5-11-18(17)19/h1-12,15,22H,13-14H2,(H,29,30)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315657

(2-(4-chloro-6-(naphthalen-2-ylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3ccccc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C26H18ClN3O2S/c27-22-15-23(28-19-13-12-16-6-1-2-8-18(16)14-19)30-26(29-22)33-24(25(31)32)21-11-5-9-17-7-3-4-10-20(17)21/h1-15,24H,(H,31,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337811

(2-(4-(biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-17-26(31-18-19-13-15-21(16-14-19)20-7-2-1-3-8-20)33-29(32-25)36-27(28(34)35)24-12-6-10-22-9-4-5-11-23(22)24/h1-17,27H,18H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 expressed in LPS-stimulated human A549 cells mitochondrial fraction assessed as conversion of PGH2 to PGE2 |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337810

(2-(4-chloro-6-(4-phenoxyphenylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Oc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O3S/c29-24-17-25(30-19-13-15-21(16-14-19)35-20-9-2-1-3-10-20)32-28(31-24)36-26(27(33)34)23-12-6-8-18-7-4-5-11-22(18)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315663
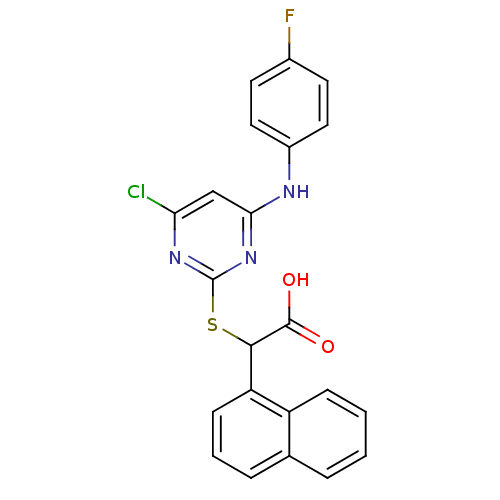
(2-(4-chloro-6-(4-fluorophenylamino)pyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(F)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C22H15ClFN3O2S/c23-18-12-19(25-15-10-8-14(24)9-11-15)27-22(26-18)30-20(21(28)29)17-7-3-5-13-4-1-2-6-16(13)17/h1-12,20H,(H,28,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315658

(2-(4-chloro-6-(2,3-dihydro-1H-inden-5-ylamino)pyri...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3CCCc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C25H20ClN3O2S/c26-21-14-22(27-18-12-11-15-6-3-8-17(15)13-18)29-25(28-21)32-23(24(30)31)20-10-4-7-16-5-1-2-9-19(16)20/h1-2,4-5,7,9-14,23H,3,6,8H2,(H,30,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337815
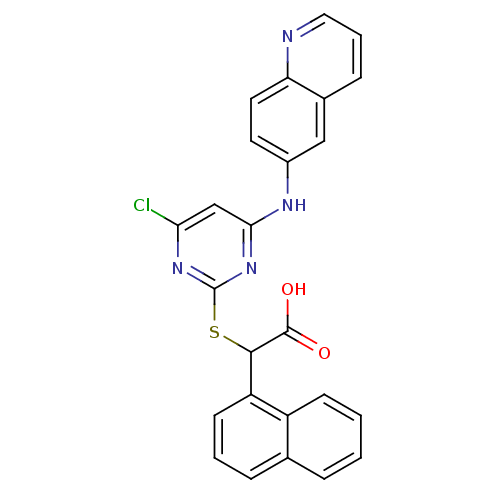
(2-(4-chloro-6-(quinolin-6-ylamino)pyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C25H17ClN4O2S/c26-21-14-22(28-17-10-11-20-16(13-17)7-4-12-27-20)30-25(29-21)33-23(24(31)32)19-9-3-6-15-5-1-2-8-18(15)19/h1-14,23H,(H,31,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315661

(2-(4-chloro-6-(3-chlorophenylamino)pyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2cccc(Cl)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C22H15Cl2N3O2S/c23-14-7-4-8-15(11-14)25-19-12-18(24)26-22(27-19)30-20(21(28)29)17-10-3-6-13-5-1-2-9-16(13)17/h1-12,20H,(H,28,29)(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50273386
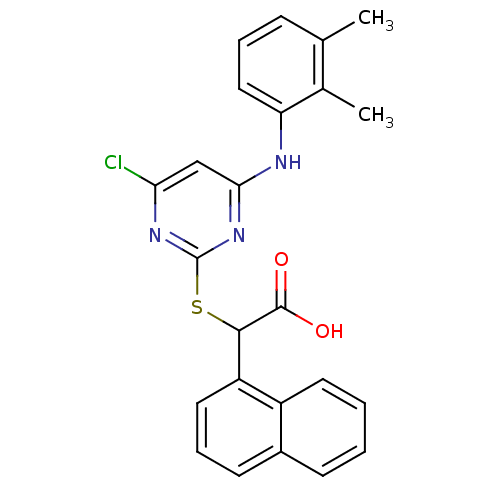
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES Cc1cccc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)c1C Show InChI InChI=1S/C24H20ClN3O2S/c1-14-7-5-12-19(15(14)2)26-21-13-20(25)27-24(28-21)31-22(23(29)30)18-11-6-9-16-8-3-4-10-17(16)18/h3-13,22H,1-2H3,(H,29,30)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337807

(2-(4-(biphenyl-3-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2cccc(c2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-13-6-12-20(16-21)18-8-2-1-3-9-18)32-28(31-24)35-26(27(33)34)23-15-7-11-19-10-4-5-14-22(19)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data