 Found 731 hits with Last Name = 'tobe' and Initial = 'm'
Found 731 hits with Last Name = 'tobe' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50444438
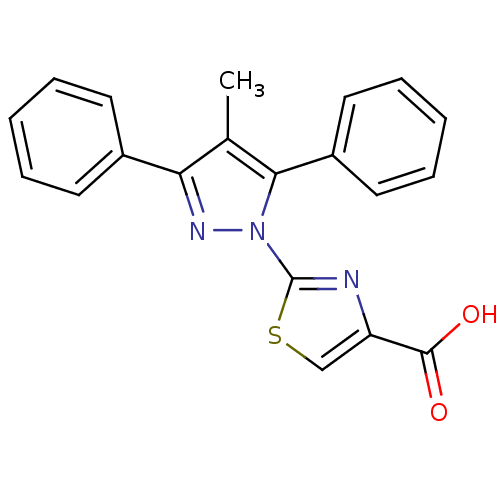
(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493969
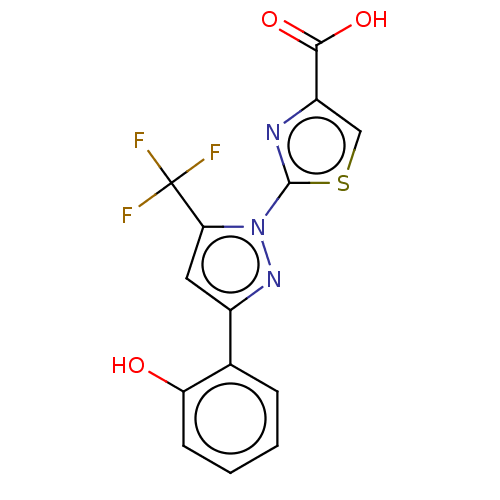
(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542127
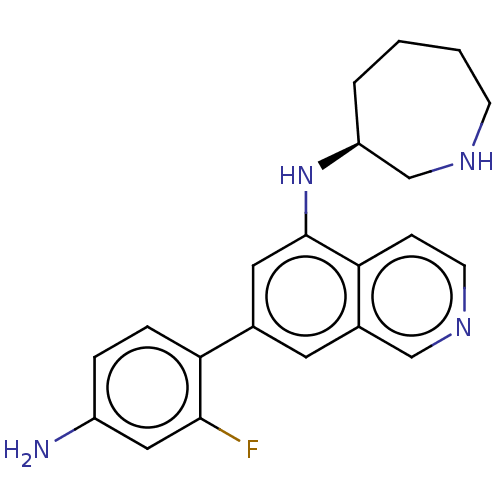
(CHEMBL4635482)Show SMILES Nc1ccc(c(F)c1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H23FN4/c22-20-11-16(23)4-5-18(20)14-9-15-12-25-8-6-19(15)21(10-14)26-17-3-1-2-7-24-13-17/h4-6,8-12,17,24,26H,1-3,7,13,23H2/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542123

(CHEMBL4636488)Show SMILES C1CNC[C@H](C1)Nc1cc(cc2cnccc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3/c1-2-5-15(6-3-1)16-11-17-13-22-10-8-19(17)20(12-16)23-18-7-4-9-21-14-18/h1-3,5-6,8,10-13,18,21,23H,4,7,9,14H2/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647518
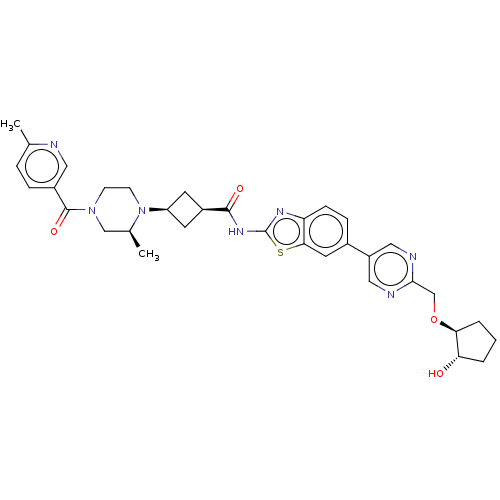
(US20240025893, Example b-04-44)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccc(C)nc1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-14.57,3.75,;-15.34,5.08,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647516
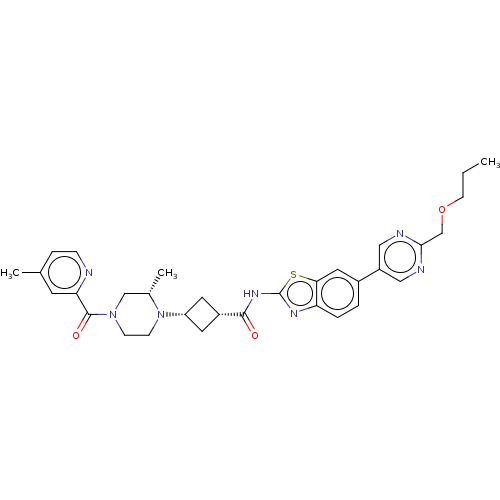
(US20240025893, Example b-04-42)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3cc(C)ccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.85,;14.15,-3.08,;12.82,-3.85,;11.49,-3.08,;10.15,-3.85,;8.82,-3.08,;7.49,-3.85,;6.15,-3.08,;6.15,-1.54,;7.49,-.77,;8.82,-1.54,;4.82,-.77,;4.82,.77,;3.48,1.54,;2.15,.77,;.69,1.25,;-.22,,;-1.76,,;-2.53,-1.33,;-1.76,-2.67,;-4.07,-1.33,;-5.16,-.24,;-6.25,-1.33,;-5.16,-2.42,;-7.79,-1.33,;-8.56,,;-10.11,,;-10.87,-1.33,;-10.11,-2.67,;-8.56,-2.67,;-7.79,-4,;-12.41,-1.33,;-13.18,-2.67,;-13.18,,;-12.41,1.33,;-13.18,2.67,;-12.41,4,;-14.72,2.67,;-15.49,1.33,;-14.72,,;.69,-1.25,;2.15,-.77,;3.48,-1.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542116

(CHEMBL4646995)Show InChI InChI=1S/C20H22N4/c21-17-5-3-14(4-6-17)15-10-16-12-23-9-7-19(16)20(11-15)24-18-2-1-8-22-13-18/h3-7,9-12,18,22,24H,1-2,8,13,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647522
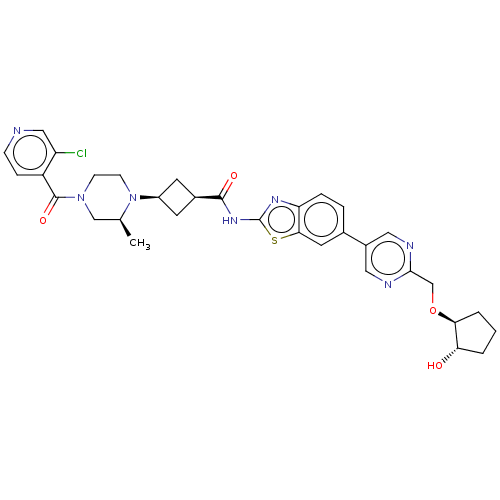
(US20240025893, Example b-04-48)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccncc1Cl |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.25,;-8.41,-.92,;-9.96,-.92,;-10.72,.41,;-9.96,1.75,;-8.41,1.75,;-7.64,.41,;-6.1,.41,;-5.01,1.5,;-3.92,.41,;-5.01,-.68,;-2.38,.41,;-1.61,-.92,;-1.61,1.75,;-.07,1.75,;.83,2.99,;2.3,2.52,;3.63,3.29,;4.97,2.52,;4.97,.98,;3.63,.21,;2.3,.98,;.83,.5,;6.3,.21,;6.3,-1.33,;7.63,-2.1,;8.97,-1.33,;10.3,-2.1,;11.63,-1.33,;12.97,-2.1,;14.43,-1.63,;15.34,-2.87,;14.43,-4.12,;12.97,-3.64,;11.63,-4.41,;8.97,.21,;7.63,.98,;-12.26,.41,;-13.03,-.92,;-13.03,1.75,;-12.26,3.08,;-13.03,4.41,;-14.57,4.41,;-15.34,3.08,;-14.57,1.75,;-15.34,.41,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647521

(US20240025893, Example b-04-47)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cc(C)ccn1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-12.26,5.08,;-14.57,3.75,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647520
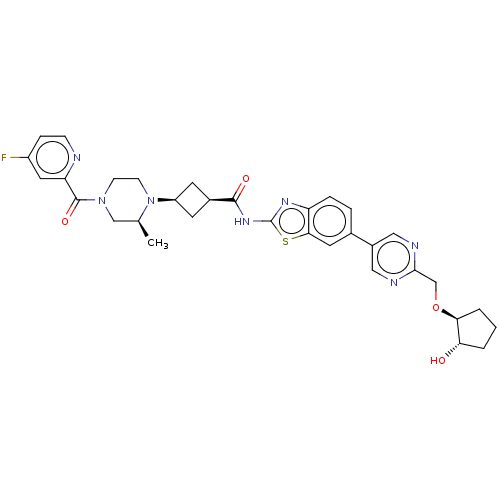
(US20240025893, Example b-04-46)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cc(F)ccn1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-12.26,5.08,;-14.57,3.75,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50449137
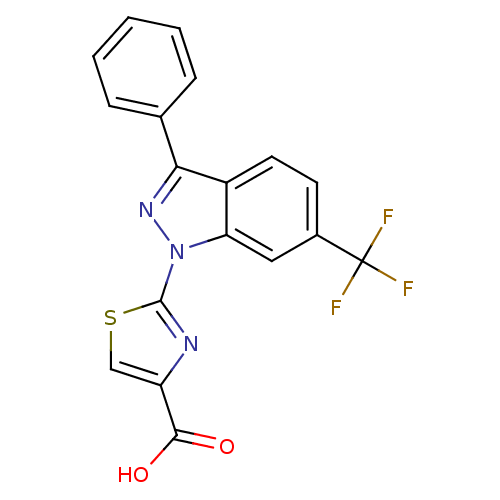
(CHEMBL3127163)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(cc12)C(F)(F)F Show InChI InChI=1S/C18H10F3N3O2S/c19-18(20,21)11-6-7-12-14(8-11)24(17-22-13(9-27-17)16(25)26)23-15(12)10-4-2-1-3-5-10/h1-9H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542119
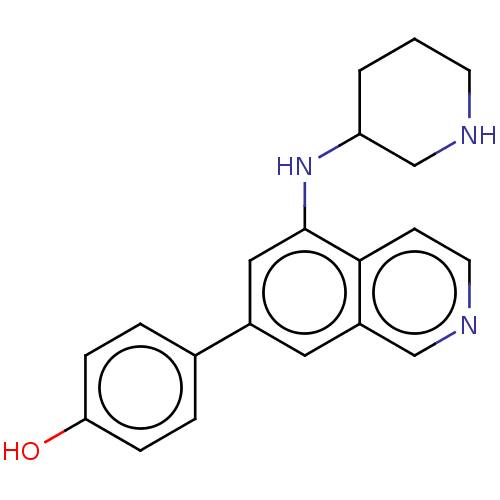
(CHEMBL4644939)Show InChI InChI=1S/C20H21N3O/c24-18-5-3-14(4-6-18)15-10-16-12-22-9-7-19(16)20(11-15)23-17-2-1-8-21-13-17/h3-7,9-12,17,21,23-24H,1-2,8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647517
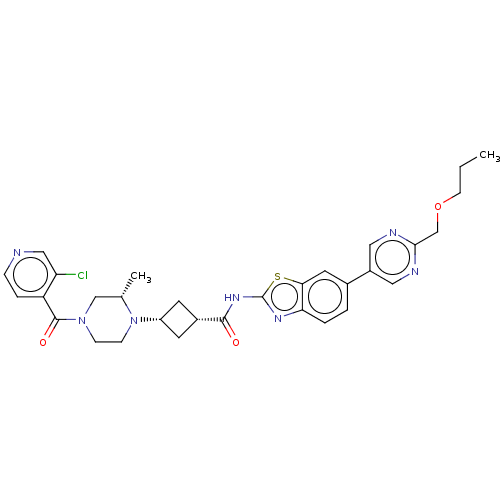
(US20240025893, Example b-04-43)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3ccncc3Cl)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.18,;14.15,-2.41,;12.82,-3.18,;11.49,-2.41,;10.15,-3.18,;8.82,-2.41,;7.49,-3.18,;6.15,-2.41,;6.15,-.87,;7.49,-.1,;8.82,-.87,;4.82,-.1,;4.82,1.44,;3.48,2.21,;2.15,1.44,;.69,1.91,;-.22,.67,;-1.76,.67,;-2.53,-.67,;-1.76,-2,;-4.07,-.67,;-5.16,.42,;-6.25,-.67,;-5.16,-1.76,;-7.79,-.67,;-8.56,.67,;-10.11,.67,;-10.87,-.67,;-10.11,-2,;-8.56,-2,;-7.79,-3.33,;-12.41,-.67,;-13.18,-2,;-13.18,.67,;-12.41,2,;-13.18,3.33,;-14.72,3.33,;-15.49,2,;-14.72,.67,;-15.49,-.67,;.69,-.58,;2.15,-.1,;3.48,-.87,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647519

(US20240025893, Example b-04-45)Show SMILES COc1ccc(cn1)C(=O)N1CCN([C@H]2C[C@H](C2)C(=O)Nc2nc3ccc(cc3s2)-c2cnc(CO[C@H]3CCC[C@@H]3O)nc2)[C@@H](C)C1 |r,wU:36.39,14.14,16.19,44.50,wD:40.45,(-14.35,6.26,;-15.34,5.08,;-14.57,3.75,;-13.03,3.75,;-12.26,2.41,;-13.03,1.08,;-14.57,1.08,;-15.34,2.41,;-12.26,-.25,;-13.03,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-8.41,-1.59,;-7.64,-2.92,;-9.96,-1.59,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647513
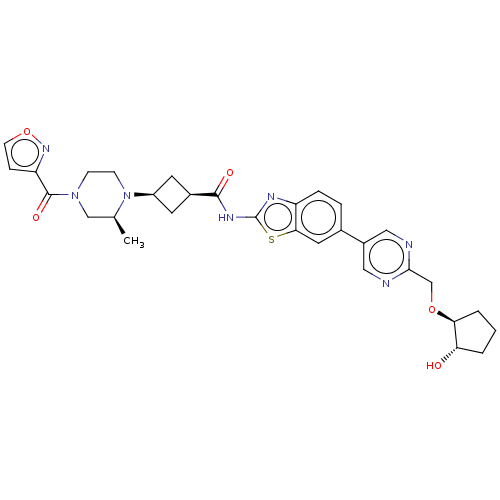
(US20240025893, Example b-04-39)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccon1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.79,-2.11,;-8.56,-.77,;-10.1,-.77,;-10.87,.56,;-10.1,1.9,;-8.56,1.9,;-7.79,.56,;-6.25,.56,;-5.16,1.65,;-4.07,.56,;-5.16,-.53,;-2.53,.56,;-1.76,-.77,;-1.76,1.9,;-.22,1.9,;.69,3.14,;2.15,2.67,;3.49,3.44,;4.82,2.67,;4.82,1.13,;3.49,.36,;2.15,1.13,;.69,.65,;6.15,.36,;6.15,-1.18,;7.49,-1.95,;8.82,-1.18,;10.15,-1.95,;11.49,-1.18,;12.82,-1.95,;14.29,-1.48,;15.19,-2.72,;14.29,-3.97,;12.82,-3.49,;11.49,-4.26,;8.82,.36,;7.49,1.13,;-12.41,.56,;-13.18,-.77,;-13.18,1.9,;-12.7,3.36,;-13.95,4.26,;-15.19,3.36,;-14.72,1.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647515

(US20240025893, Example b-04-41)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3cc(F)ccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.85,;14.15,-3.08,;12.82,-3.85,;11.49,-3.08,;10.15,-3.85,;8.82,-3.08,;7.49,-3.85,;6.15,-3.08,;6.15,-1.54,;7.49,-.77,;8.82,-1.54,;4.82,-.77,;4.82,.77,;3.48,1.54,;2.15,.77,;.69,1.25,;-.22,,;-1.76,,;-2.53,-1.33,;-1.76,-2.67,;-4.07,-1.33,;-5.16,-.24,;-6.25,-1.33,;-5.16,-2.42,;-7.79,-1.33,;-8.56,,;-10.11,,;-10.87,-1.33,;-10.11,-2.67,;-8.56,-2.67,;-7.79,-4,;-12.41,-1.33,;-13.18,-2.67,;-13.18,,;-12.41,1.33,;-13.18,2.67,;-12.41,4,;-14.72,2.67,;-15.49,1.33,;-14.72,,;.69,-1.25,;2.15,-.77,;3.48,-1.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276250
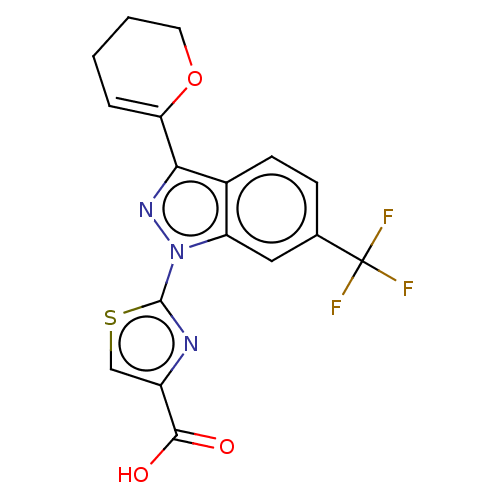
(CHEMBL4126319)Show SMILES OC(=O)c1csc(n1)-n1nc(C2=CCCCO2)c2ccc(cc12)C(F)(F)F |t:12| Show InChI InChI=1S/C17H12F3N3O3S/c18-17(19,20)9-4-5-10-12(7-9)23(16-21-11(8-27-16)15(24)25)22-14(10)13-3-1-2-6-26-13/h3-5,7-8H,1-2,6H2,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647498
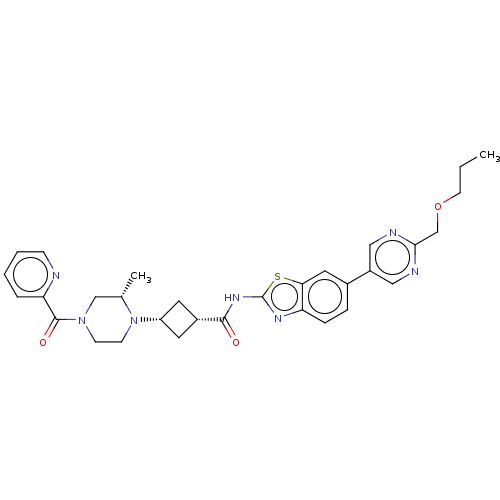
(US20240025893, Example b-04-24)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3ccccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.18,;14.15,-2.41,;12.82,-3.18,;11.49,-2.41,;10.15,-3.18,;8.82,-2.41,;7.49,-3.18,;6.15,-2.41,;6.15,-.87,;7.49,-.1,;8.82,-.87,;4.82,-.1,;4.82,1.44,;3.48,2.21,;2.15,1.44,;.69,1.91,;-.22,.67,;-1.76,.67,;-2.53,-.67,;-1.76,-2,;-4.07,-.67,;-5.16,.42,;-6.25,-.67,;-5.16,-1.76,;-7.79,-.67,;-8.56,.67,;-10.11,.67,;-10.87,-.67,;-10.11,-2,;-8.56,-2,;-7.79,-3.33,;-12.41,-.67,;-13.18,-2,;-13.18,.67,;-12.41,2,;-13.18,3.33,;-14.72,3.33,;-15.49,2,;-14.72,.67,;.69,-.58,;2.15,-.1,;3.48,-.87,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634505
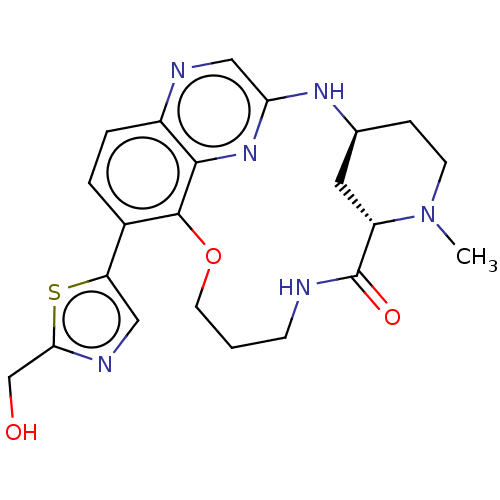
(US20230357271, Example a-01-11)Show SMILES CN1CC[C@H]2C[C@H]1C(=O)NCCCOc1c(ccc3ncc(N2)nc13)-c1cnc(CO)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542128
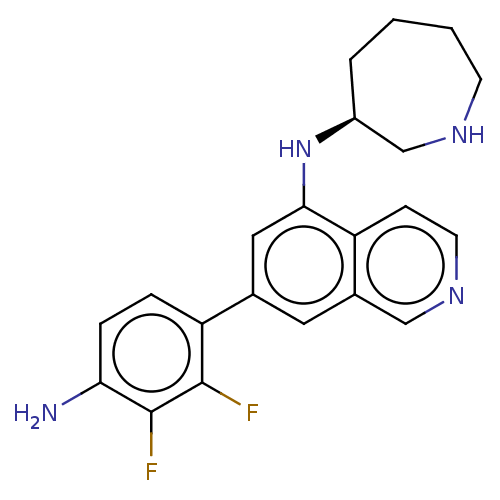
(CHEMBL4636337)Show SMILES Nc1ccc(c(F)c1F)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H22F2N4/c22-20-17(4-5-18(24)21(20)23)13-9-14-11-26-8-6-16(14)19(10-13)27-15-3-1-2-7-25-12-15/h4-6,8-11,15,25,27H,1-3,7,12,24H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647464

(US20240025893, Example b-02-86)Show SMILES O[C@H]1CCC[C@@H]1OCc1ncc(cn1)-c1ccc2nc(NC(=O)C3CC(C3)NCc3nccs3)sc2c1 |r,wU:5.6,wD:1.0,(10.12,-3.85,;11.46,-3.08,;12.92,-3.56,;13.83,-2.31,;12.92,-1.06,;11.46,-1.54,;10.12,-.77,;8.79,-1.54,;7.46,-.77,;6.12,-1.54,;4.79,-.77,;4.79,.77,;6.12,1.54,;7.46,.77,;3.46,1.54,;3.46,3.08,;2.12,3.85,;.79,3.08,;-.68,3.56,;-1.58,2.31,;-3.12,2.31,;-3.89,.98,;-3.12,-.36,;-5.43,.98,;-6.52,2.07,;-7.61,.98,;-6.52,-.11,;-9.15,.98,;-9.92,-.36,;-11.46,-.36,;-12.36,-1.6,;-13.83,-1.13,;-13.83,.41,;-12.36,.89,;-.68,1.06,;.79,1.54,;2.12,.77,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.803 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647511
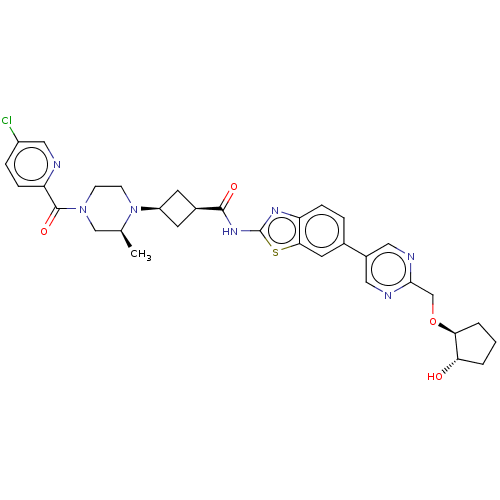
(US20240025893, Example b-04-37)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccc(Cl)cn1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-14.57,3.75,;-15.34,5.08,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634536
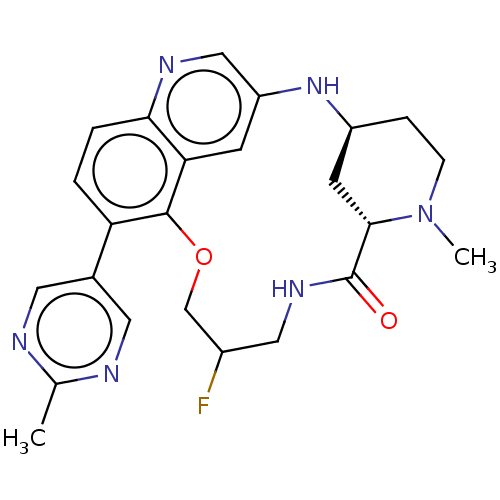
(US20230357271, Example a-02-16)Show SMILES CN1CC[C@H]2C[C@H]1C(=O)NCC(F)COc1c(ccc3ncc(N2)cc13)-c1cnc(C)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647569

(US20240025893, Example d-03-06)Show SMILES O[C@H]1CCC[C@@H]1OCc1ncc(cn1)-c1ccc2nc(NC(=O)C3CC4(C3)CCN(CC4)C(=O)c3ccccn3)sc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.849 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647512

(US20240025893, Example b-04-38)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cncnc1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.25,;-8.41,-.92,;-9.96,-.92,;-10.72,.41,;-9.96,1.75,;-8.41,1.75,;-7.64,.41,;-6.1,.41,;-5.01,1.5,;-3.92,.41,;-5.01,-.68,;-2.38,.41,;-1.61,-.92,;-1.61,1.75,;-.07,1.75,;.83,2.99,;2.3,2.52,;3.63,3.29,;4.97,2.52,;4.97,.98,;3.63,.21,;2.3,.98,;.83,.5,;6.3,.21,;6.3,-1.33,;7.63,-2.1,;8.97,-1.33,;10.3,-2.1,;11.63,-1.33,;12.97,-2.1,;14.43,-1.63,;15.34,-2.87,;14.43,-4.12,;12.97,-3.64,;11.63,-4.41,;8.97,.21,;7.63,.98,;-12.26,.41,;-13.03,-.92,;-13.03,1.75,;-12.26,3.08,;-13.03,4.41,;-14.57,4.41,;-15.34,3.08,;-14.57,1.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647570

(US20240025893, Example d-03-07)Show SMILES O[C@H]1CCC[C@@H]1OCc1ncc(cn1)-c1ccc2nc(NC(=O)C3CC4(C3)CCN(CC4)C(=O)c3cccnc3)sc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.852 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
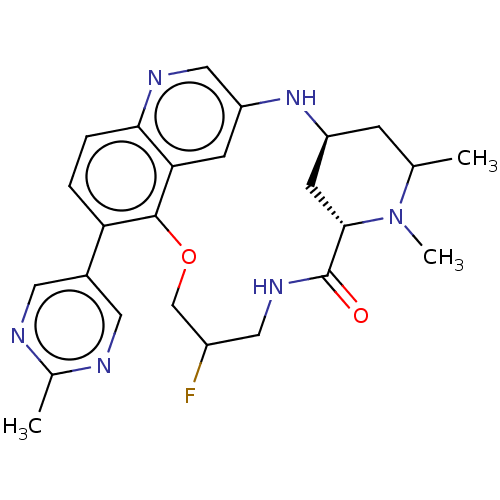
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634549
(US20230357271, Example a-02-09)Show SMILES CC1C[C@H]2C[C@H](N1C)C(=O)NCC(F)COc1c(ccc3ncc(N2)cc13)-c1cnc(C)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647499

(US20240025893, Example b-04-25)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)C3CCOC3)sc2c1 |r,wU:22.25,20.20,29.32,(15.34,-3.03,;14.01,-2.26,;12.67,-3.03,;11.34,-2.26,;10.01,-3.03,;8.67,-2.26,;7.34,-3.03,;6,-2.26,;6,-.72,;7.34,.05,;8.67,-.72,;4.67,.05,;4.67,1.59,;3.34,2.36,;2,1.59,;.54,2.06,;-.37,.82,;-1.91,.82,;-2.68,-.52,;-1.91,-1.85,;-4.22,-.52,;-5.31,.57,;-6.39,-.52,;-5.31,-1.61,;-7.93,-.52,;-8.7,.82,;-10.25,.82,;-11.01,-.52,;-10.25,-1.85,;-8.7,-1.85,;-7.93,-3.19,;-12.55,-.52,;-13.32,-1.85,;-13.32,.82,;-12.85,2.28,;-14.09,3.19,;-15.34,2.28,;-14.86,.82,;.54,-.43,;2,.05,;3.34,-.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647604

(US20240025893, Example e-02-23)Show SMILES CCCOCc1ncc(cn1)-c1ccn2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3cc(C)nc(C)c3)cc2c1 |r,wU:20.20,22.25,29.32,(16.26,-3.85,;14.92,-3.08,;13.59,-3.85,;12.26,-3.08,;10.92,-3.85,;9.59,-3.08,;8.26,-3.85,;6.92,-3.08,;6.92,-1.54,;8.26,-.77,;9.59,-1.54,;5.59,-.77,;5.59,.77,;4.25,1.54,;2.92,.77,;1.46,1.25,;.55,,;-.99,,;-1.76,-1.33,;-.99,-2.67,;-3.3,-1.33,;-4.39,-.24,;-5.48,-1.33,;-4.39,-2.42,;-7.02,-1.33,;-7.79,,;-9.33,,;-10.1,-1.33,;-9.33,-2.67,;-7.79,-2.67,;-7.02,-4,;-11.64,-1.33,;-12.41,-2.67,;-12.41,,;-11.64,1.33,;-12.41,2.67,;-11.64,4,;-13.95,2.67,;-14.72,1.33,;-16.26,1.33,;-13.95,,;1.46,-1.25,;2.92,-.77,;4.25,-1.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647514
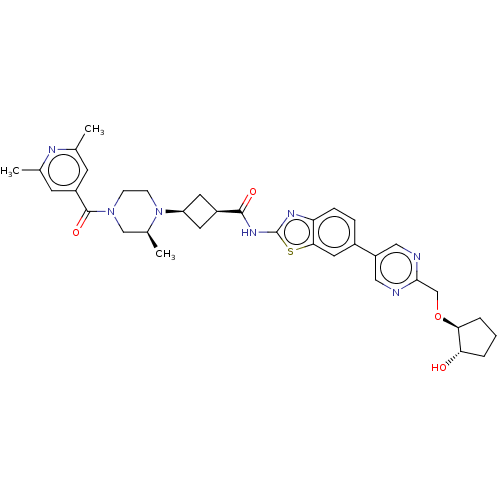
(US20240025893, Example b-04-40)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cc(C)nc(C)c1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-6.87,-2.92,;-7.64,-1.59,;-9.19,-1.59,;-9.95,-.25,;-9.19,1.08,;-7.64,1.08,;-6.87,-.25,;-5.33,-.25,;-4.24,.83,;-3.15,-.25,;-4.24,-1.34,;-1.61,-.25,;-.84,-1.59,;-.84,1.08,;.7,1.08,;1.6,2.33,;3.07,1.85,;4.4,2.62,;5.74,1.85,;5.74,.31,;4.4,-.46,;3.07,.31,;1.6,-.17,;7.07,-.46,;7.07,-2,;8.4,-2.77,;9.74,-2,;11.07,-2.77,;12.4,-2,;13.74,-2.77,;15.2,-2.29,;16.11,-3.54,;15.2,-4.79,;13.74,-4.31,;12.4,-5.08,;9.74,-.46,;8.4,.31,;-11.49,-.25,;-12.26,-1.59,;-12.26,1.08,;-11.49,2.41,;-12.26,3.75,;-11.49,5.08,;-13.8,3.75,;-14.57,2.41,;-16.11,2.41,;-13.8,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634353
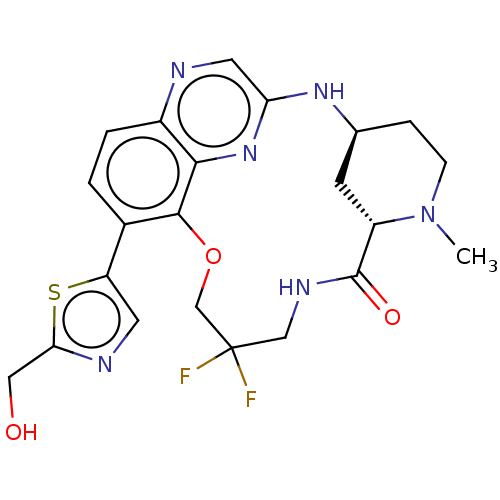
(US20230357271, Example a-01-22)Show SMILES CN1CC[C@H]2C[C@H]1C(=O)NCC(F)(F)COc1c(ccc3ncc(N2)nc13)-c1cnc(CO)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634349

(US20230357271, Example a-01-02)Show SMILES CN1CC[C@H]2C[C@H]1C(=O)NCC1(CC1)COc1c(ccc3ncc(N2)nc13)-c1cnc(CO)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542132

(CHEMBL4632523)Show SMILES Nc1ccc(cn1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C20H23N5/c21-20-5-4-14(12-24-20)15-9-16-11-23-8-6-18(16)19(10-15)25-17-3-1-2-7-22-13-17/h4-6,8-12,17,22,25H,1-3,7,13H2,(H2,21,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647510

(US20240025893, Example b-04-36)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1coc(C)n1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.05,-2.11,;-7.82,-.77,;-9.37,-.77,;-10.13,.56,;-9.37,1.9,;-7.82,1.9,;-7.05,.56,;-5.51,.56,;-4.42,1.65,;-3.34,.56,;-4.42,-.53,;-1.8,.56,;-1.03,-.77,;-1.03,1.9,;.51,1.9,;1.42,3.14,;2.88,2.67,;4.22,3.44,;5.55,2.67,;5.55,1.13,;4.22,.36,;2.88,1.13,;1.42,.65,;6.89,.36,;6.89,-1.18,;8.22,-1.95,;9.55,-1.18,;10.89,-1.95,;12.22,-1.18,;13.55,-1.95,;15.02,-1.48,;15.92,-2.72,;15.02,-3.97,;13.55,-3.49,;12.22,-4.26,;9.55,.36,;8.22,1.13,;-11.67,.56,;-12.44,-.77,;-12.44,1.9,;-11.97,3.36,;-13.21,4.26,;-14.46,3.36,;-15.92,3.84,;-13.98,1.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM634528
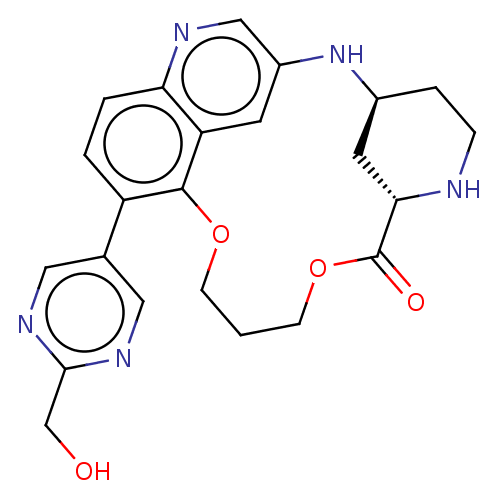
(US20230357271, Example a-02-12)Show SMILES OCc1ncc(cn1)-c1ccc2ncc3N[C@H]4CCN[C@@H](C4)C(=O)OCCCOc1c2c3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data