

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heparanase (Homo sapiens (Human)) | BDBM50466629 (CHEMBL4280766) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50587990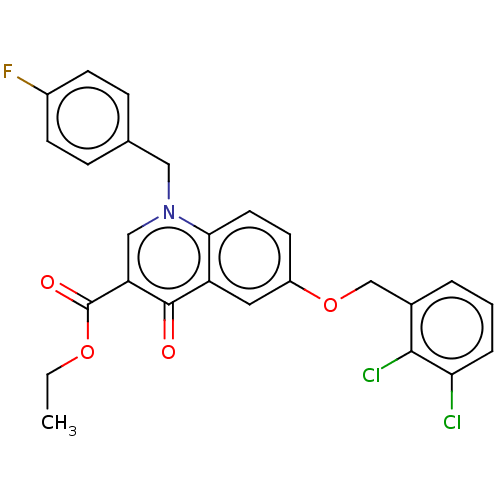 (CHEMBL5182942) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466636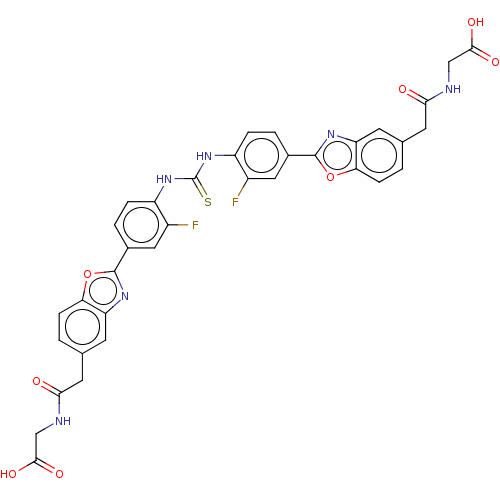 (CHEMBL4286441) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50506597 (CHEMBL4576477) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466651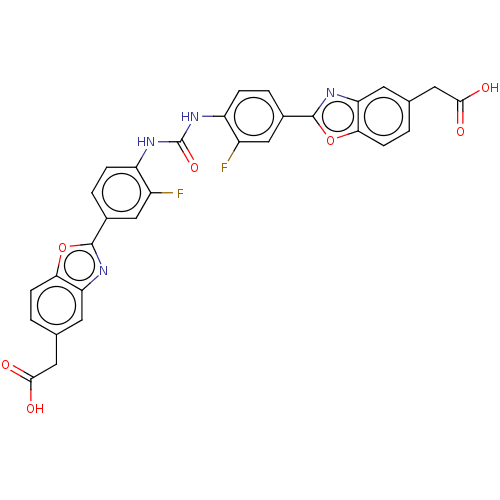 (CHEMBL4283251) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33411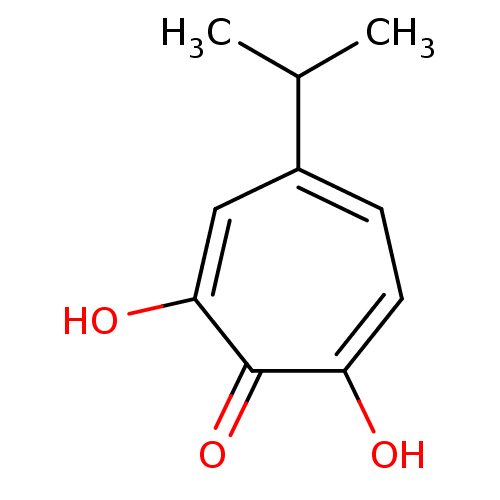 (β-Thujaplicinol | hydroxytropolone, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50540702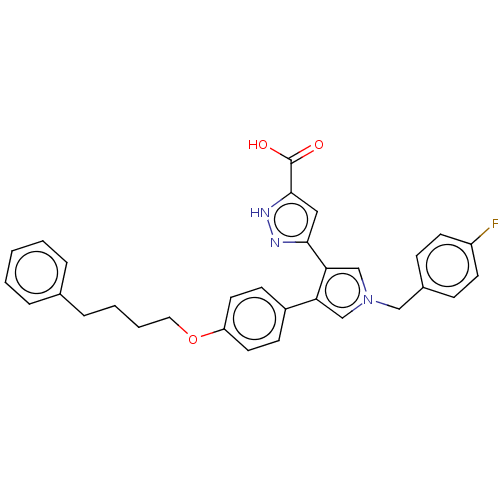 (CHEMBL4641618) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... | ACS Med Chem Lett 11: 798-805 (2020) Article DOI: 10.1021/acsmedchemlett.9b00617 BindingDB Entry DOI: 10.7270/Q2T1576W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466634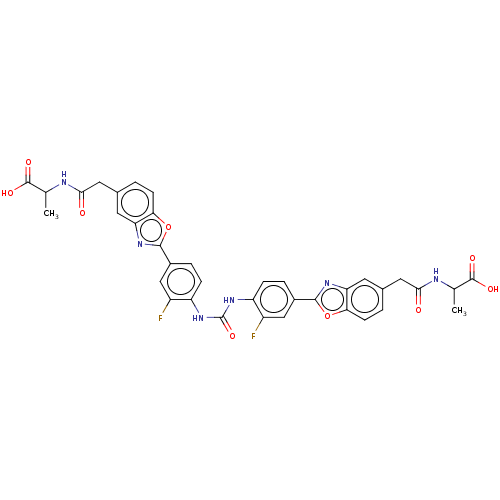 (CHEMBL4290499) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50175811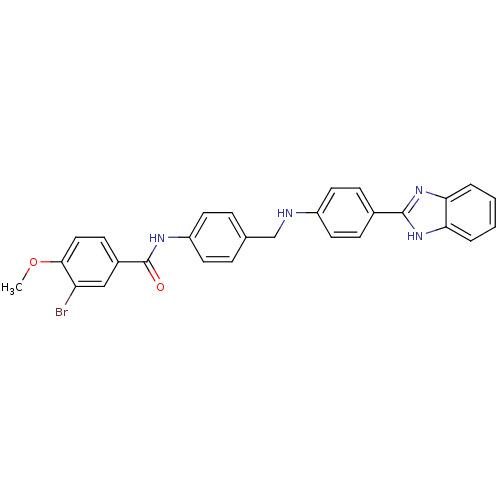 (CHEMBL200215 | N-(4-{[4-(1H-benzoimidazol-2-yl)phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529951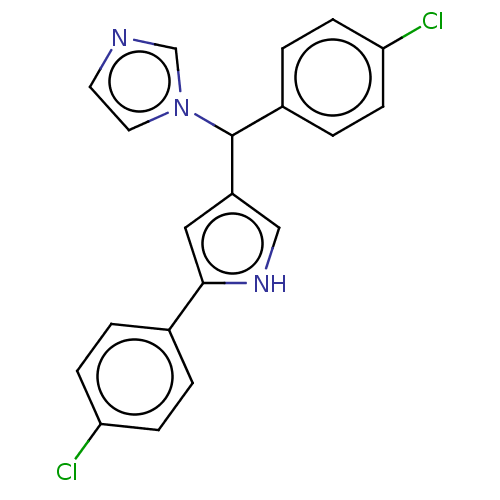 (CHEMBL4468270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529951 (CHEMBL4468270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50587966 (CHEMBL5188823) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50165612 ((2-{4-[3-((E)-4-Bromo-phenyl)-acryloylamino]-pheny...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466633 (CHEMBL4283311) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50175932 (1,3-bis-[4-(5,6-dimethyl-1H-benzoimidazol-2-yl)phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466645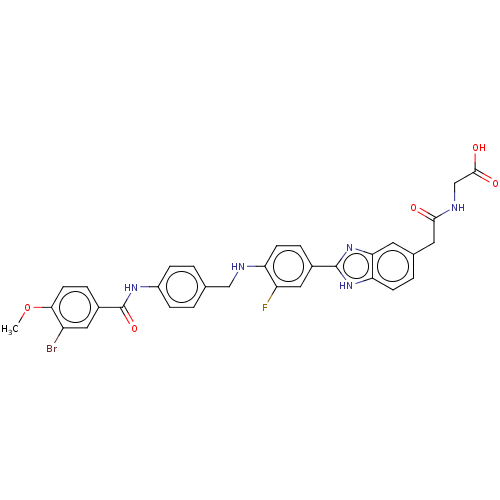 (CHEMBL4294823) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466645 (CHEMBL4294823) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466635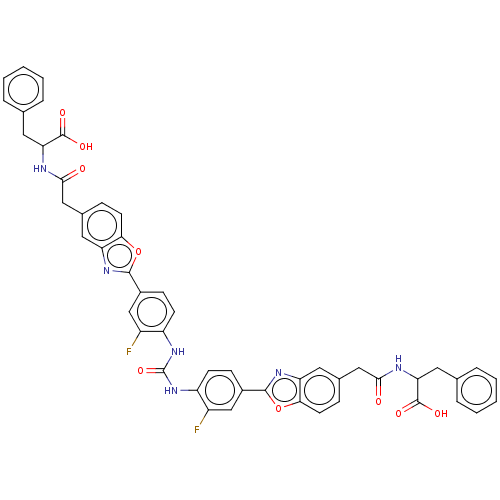 (CHEMBL4279847) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529952 (CHEMBL315874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529952 (CHEMBL315874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50506608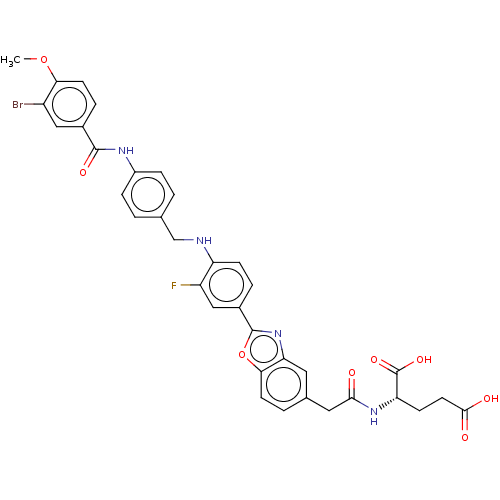 (CHEMBL4564079) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466626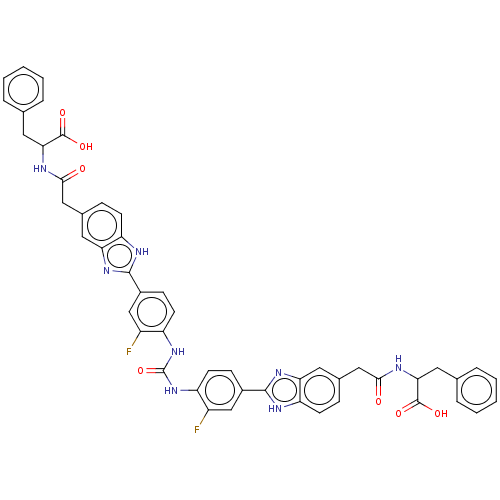 (CHEMBL4289864) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529950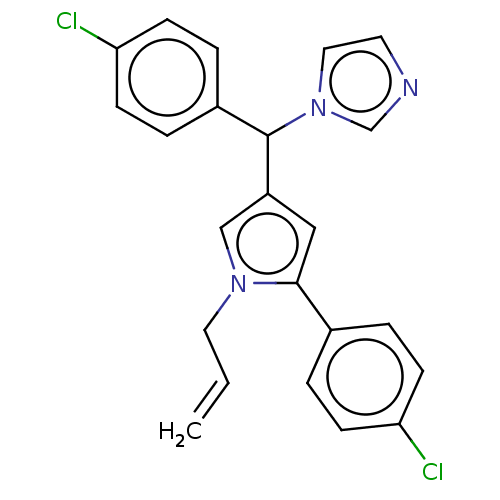 (CHEMBL4551139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529949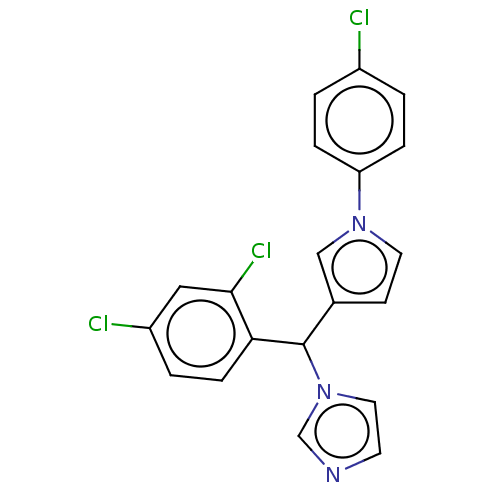 (CHEMBL4438726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529949 (CHEMBL4438726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529950 (CHEMBL4551139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466632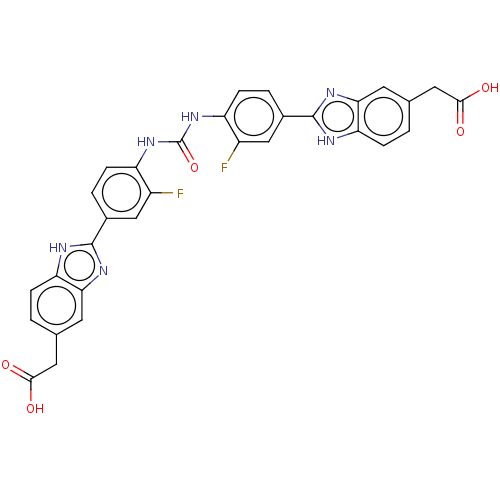 (CHEMBL4291586) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466652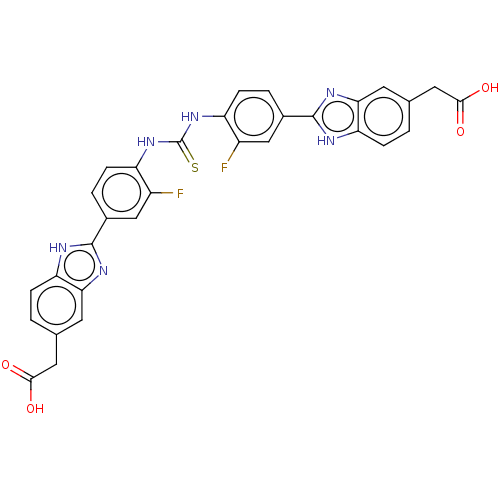 (CHEMBL4293911) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529948 (CHEMBL4569370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529948 (CHEMBL4569370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50506607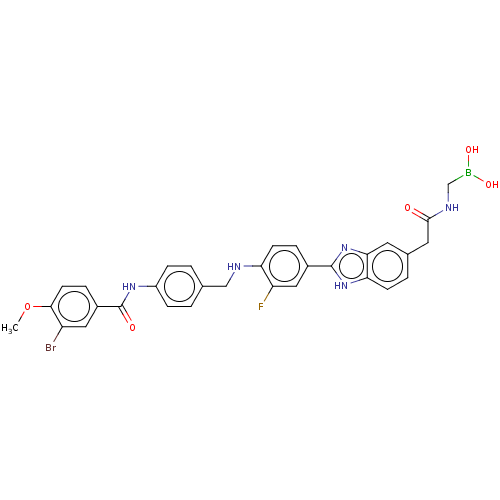 (CHEMBL4446276) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529950 (CHEMBL4551139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529950 (CHEMBL4551139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50529952 (CHEMBL315874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50529952 (CHEMBL315874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50506598 (CHEMBL4455617) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529951 (CHEMBL4468270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529951 (CHEMBL4468270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466623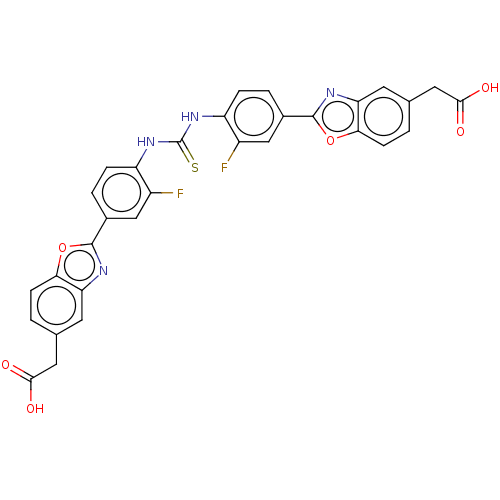 (CHEMBL4295004) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50587988 (CHEMBL5172754) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50587969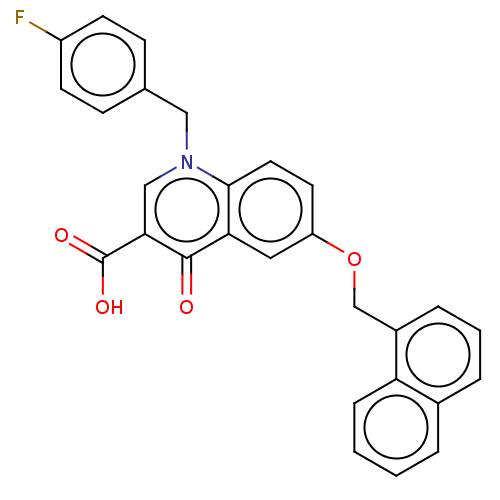 (CHEMBL5173288) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50506606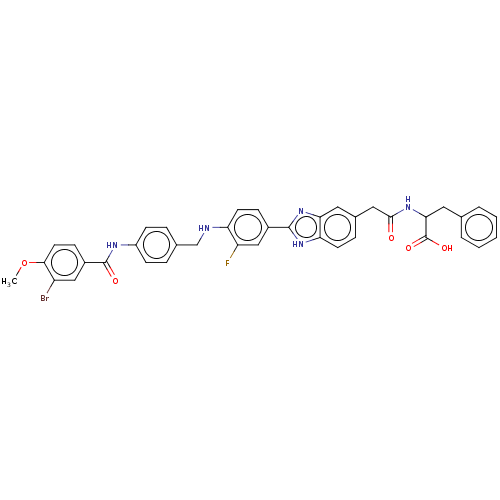 (CHEMBL4467480) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry | J Med Chem 61: 6918-6936 (2018) Article DOI: 10.1021/acs.jmedchem.8b00908 BindingDB Entry DOI: 10.7270/Q2MS3X1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50540705 (CHEMBL4641010) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... | ACS Med Chem Lett 11: 798-805 (2020) Article DOI: 10.1021/acsmedchemlett.9b00617 BindingDB Entry DOI: 10.7270/Q2T1576W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466640 (CHEMBL4282189) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 199 total ) | Next | Last >> |