 Found 301 hits with Last Name = 'varadi' and Initial = 'a'
Found 301 hits with Last Name = 'varadi' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50609010
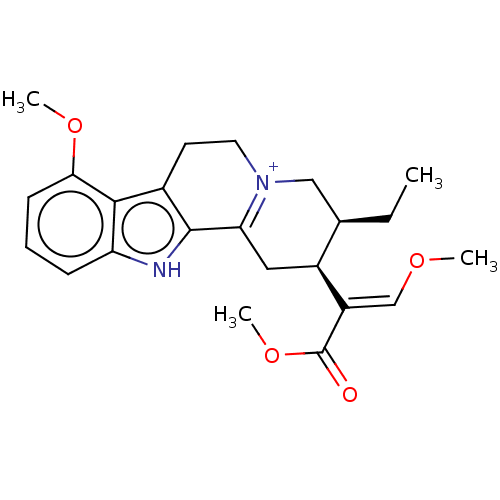
(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50519927
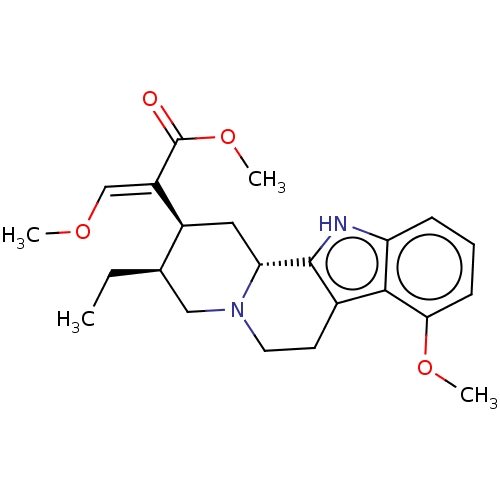
(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 649 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566317
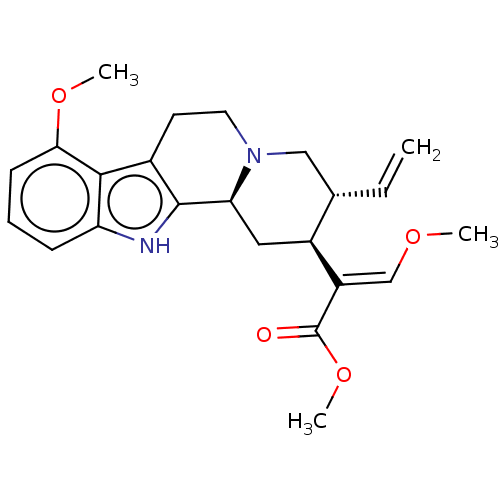
(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 666 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM249466
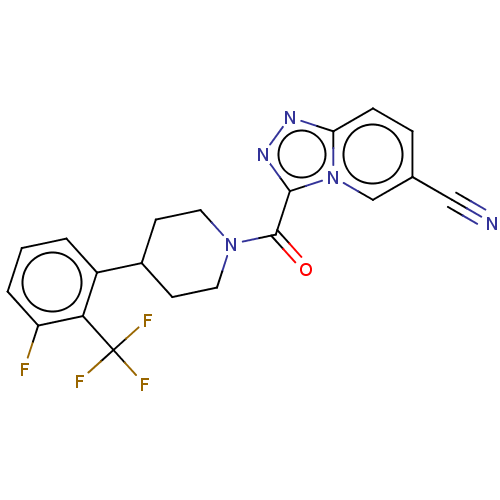
(US10072016, Compound 65 | US10407433, Compound 65 ...)Show SMILES Fc1cccc(C2CCN(CC2)C(=O)c2nnc3ccc(cn23)C#N)c1C(F)(F)F Show InChI InChI=1S/C20H15F4N5O/c21-15-3-1-2-14(17(15)20(22,23)24)13-6-8-28(9-7-13)19(30)18-27-26-16-5-4-12(10-25)11-29(16)18/h1-5,11,13H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2D6 in human liver microsomes assessed as equilibrium inhibition binding constant in presence of NADPH by LC-MS/MS an... |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609011
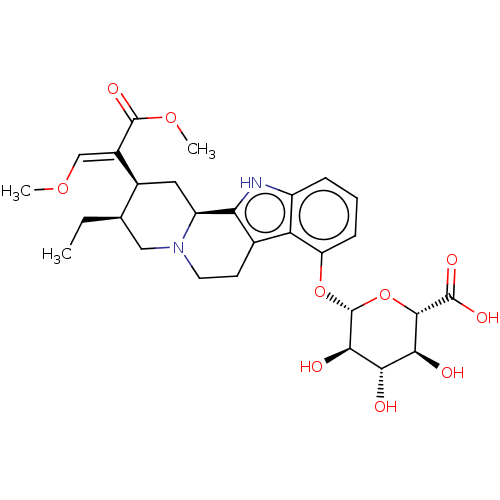
(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50501900

(CHEMBL4463039)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1nnc2ccc(cn12)C#N Show InChI InChI=1S/C20H16F3N5O/c21-20(22,23)16-4-2-1-3-15(16)14-7-9-27(10-8-14)19(29)18-26-25-17-6-5-13(11-24)12-28(17)18/h1-6,12,14H,7-10H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2D6 in human liver microsomes assessed as equilibrium inhibition binding constant in presence of NADPH by LC-MS/MS an... |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609009
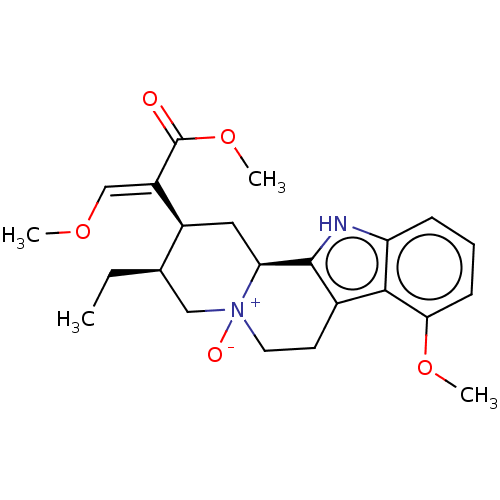
(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609009

(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609009

(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 7.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501925
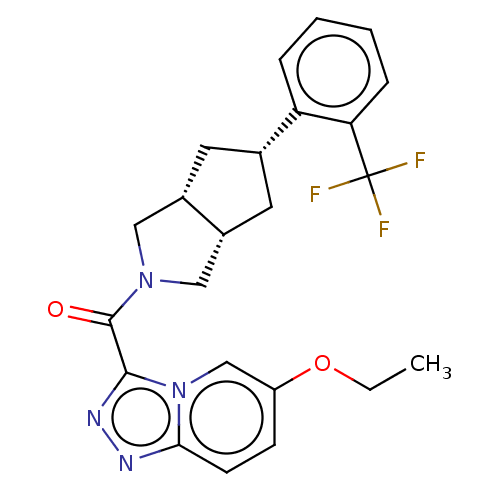
(CHEMBL4472596)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(OCC)cn12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C23H23F3N4O2/c1-2-32-17-7-8-20-27-28-21(30(20)13-17)22(31)29-11-15-9-14(10-16(15)12-29)18-5-3-4-6-19(18)23(24,25)26/h3-8,13-16H,2,9-12H2,1H3/t14-,15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501921
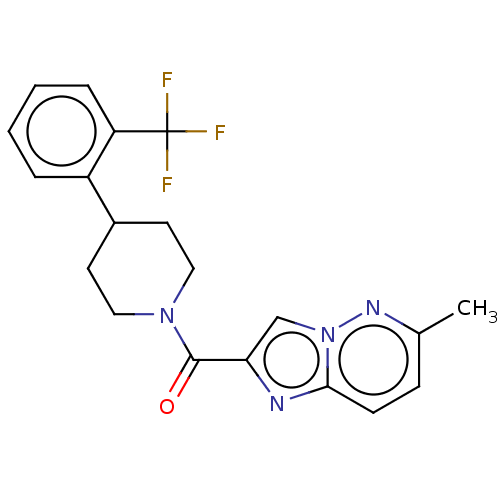
(CHEMBL4462481)Show SMILES Cc1ccc2nc(cn2n1)C(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C20H19F3N4O/c1-13-6-7-18-24-17(12-27(18)25-13)19(28)26-10-8-14(9-11-26)15-4-2-3-5-16(15)20(21,22)23/h2-7,12,14H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501914

(CHEMBL4546092)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(cn12)C(F)(F)F)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H18F6N4O/c23-21(24,25)15-5-6-18-29-30-19(32(18)11-15)20(33)31-9-13-7-12(8-14(13)10-31)16-3-1-2-4-17(16)22(26,27)28/h1-6,11-14H,7-10H2/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501899
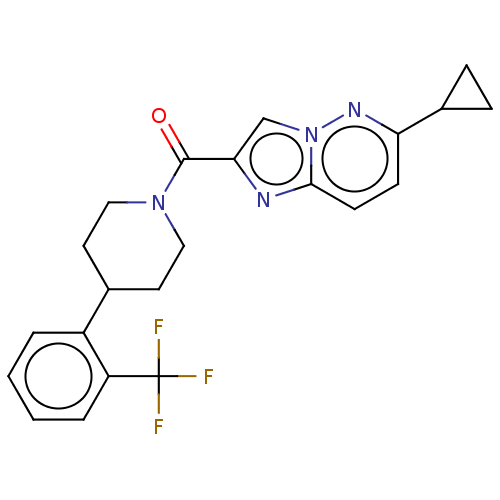
(CHEMBL4469381)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1cn2nc(ccc2n1)C1CC1 Show InChI InChI=1S/C22H21F3N4O/c23-22(24,25)17-4-2-1-3-16(17)14-9-11-28(12-10-14)21(30)19-13-29-20(26-19)8-7-18(27-29)15-5-6-15/h1-4,7-8,13-15H,5-6,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501910
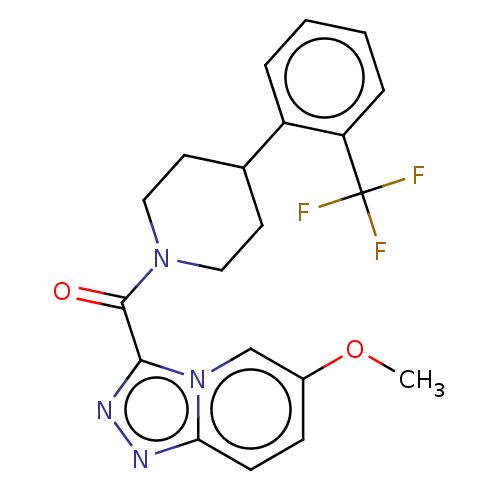
(CHEMBL4452483)Show SMILES COc1ccc2nnc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)n2c1 Show InChI InChI=1S/C20H19F3N4O2/c1-29-14-6-7-17-24-25-18(27(17)12-14)19(28)26-10-8-13(9-11-26)15-4-2-3-5-16(15)20(21,22)23/h2-7,12-13H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501929
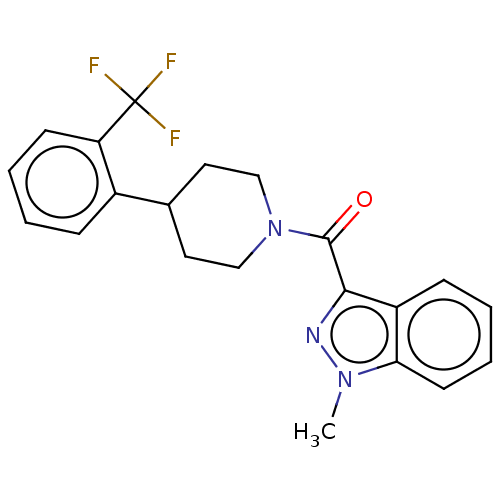
(CHEMBL4532832)Show SMILES Cn1nc(C(=O)N2CCC(CC2)c2ccccc2C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C21H20F3N3O/c1-26-18-9-5-3-7-16(18)19(25-26)20(28)27-12-10-14(11-13-27)15-6-2-4-8-17(15)21(22,23)24/h2-9,14H,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501911
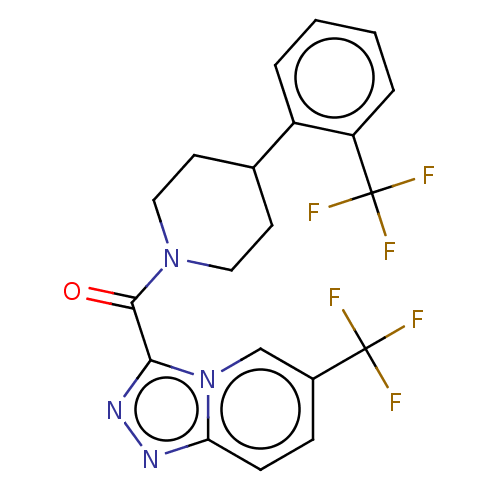
(CHEMBL4468564)Show SMILES FC(F)(F)c1ccc2nnc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)n2c1 Show InChI InChI=1S/C20H16F6N4O/c21-19(22,23)13-5-6-16-27-28-17(30(16)11-13)18(31)29-9-7-12(8-10-29)14-3-1-2-4-15(14)20(24,25)26/h1-6,11-12H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501915
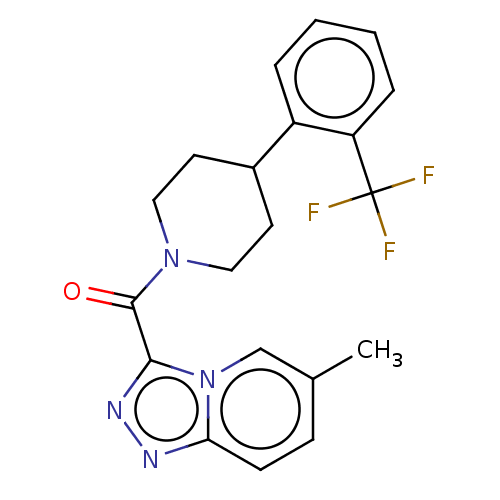
(CHEMBL4524379)Show SMILES Cc1ccc2nnc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)n2c1 Show InChI InChI=1S/C20H19F3N4O/c1-13-6-7-17-24-25-18(27(17)12-13)19(28)26-10-8-14(9-11-26)15-4-2-3-5-16(15)20(21,22)23/h2-7,12,14H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501895
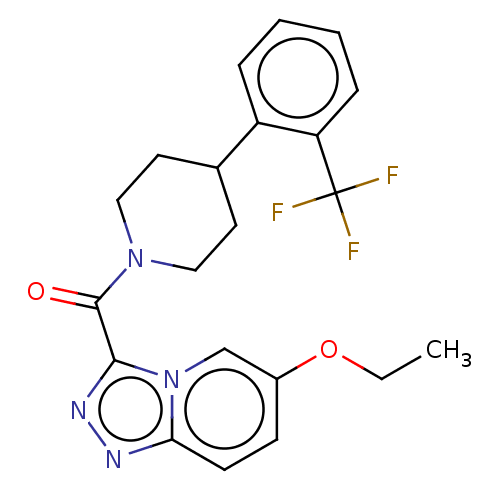
(CHEMBL4570619)Show SMILES CCOc1ccc2nnc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)n2c1 Show InChI InChI=1S/C21H21F3N4O2/c1-2-30-15-7-8-18-25-26-19(28(18)13-15)20(29)27-11-9-14(10-12-27)16-5-3-4-6-17(16)21(22,23)24/h3-8,13-14H,2,9-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501932
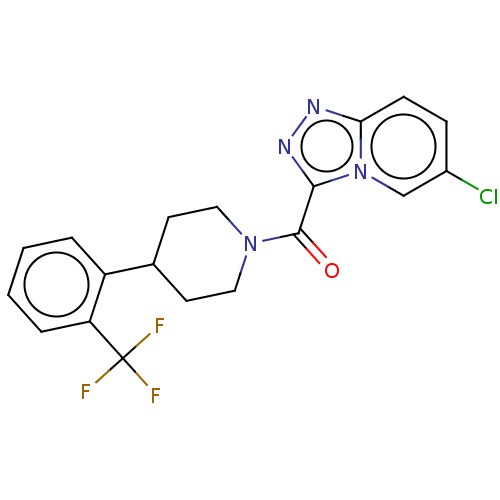
(CHEMBL4457361)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1nnc2ccc(Cl)cn12 Show InChI InChI=1S/C19H16ClF3N4O/c20-13-5-6-16-24-25-17(27(16)11-13)18(28)26-9-7-12(8-10-26)14-3-1-2-4-15(14)19(21,22)23/h1-6,11-12H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501931
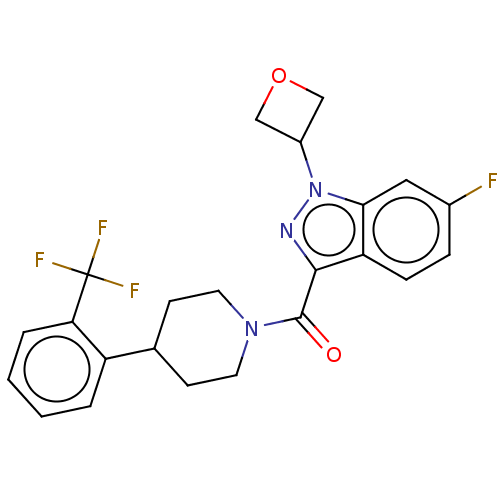
(CHEMBL4514302)Show SMILES Fc1ccc2c(nn(C3COC3)c2c1)C(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C23H21F4N3O2/c24-15-5-6-18-20(11-15)30(16-12-32-13-16)28-21(18)22(31)29-9-7-14(8-10-29)17-3-1-2-4-19(17)23(25,26)27/h1-6,11,14,16H,7-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501933
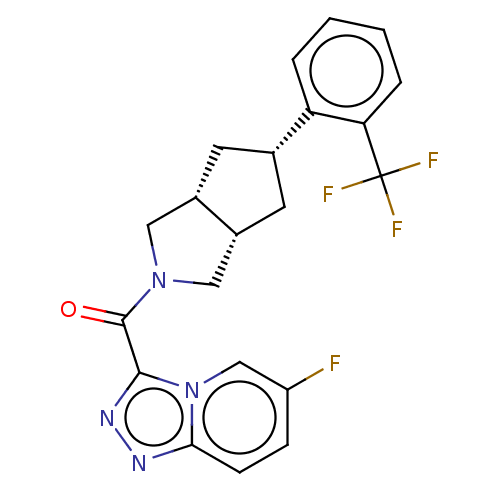
(CHEMBL4559814)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(F)cn12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C21H18F4N4O/c22-15-5-6-18-26-27-19(29(18)11-15)20(30)28-9-13-7-12(8-14(13)10-28)16-3-1-2-4-17(16)21(23,24)25/h1-6,11-14H,7-10H2/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501912

(CHEMBL4586625)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(Cl)cn12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C21H18ClF3N4O/c22-15-5-6-18-26-27-19(29(18)11-15)20(30)28-9-13-7-12(8-14(13)10-28)16-3-1-2-4-17(16)21(23,24)25/h1-6,11-14H,7-10H2/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501909
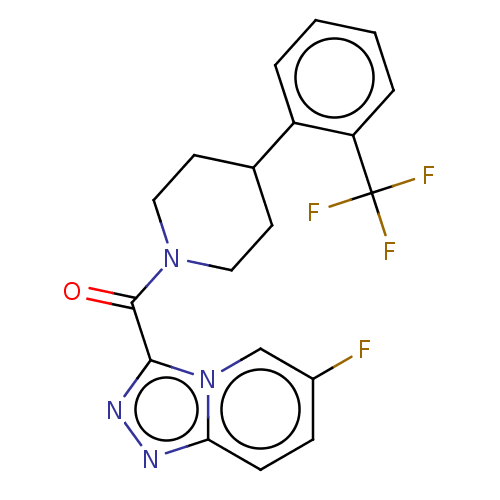
(CHEMBL4470444)Show SMILES Fc1ccc2nnc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)n2c1 Show InChI InChI=1S/C19H16F4N4O/c20-13-5-6-16-24-25-17(27(16)11-13)18(28)26-9-7-12(8-10-26)14-3-1-2-4-15(14)19(21,22)23/h1-6,11-12H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501930
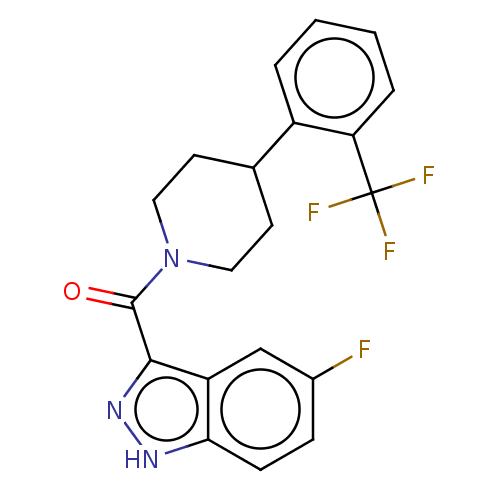
(CHEMBL4439759)Show SMILES Fc1ccc2[nH]nc(C(=O)N3CCC(CC3)c3ccccc3C(F)(F)F)c2c1 Show InChI InChI=1S/C20H17F4N3O/c21-13-5-6-17-15(11-13)18(26-25-17)19(28)27-9-7-12(8-10-27)14-3-1-2-4-16(14)20(22,23)24/h1-6,11-12H,7-10H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501902

(CHEMBL4457350)Show SMILES Fc1ccc2c(n[nH]c2c1)C(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C20H17F4N3O/c21-13-5-6-15-17(11-13)25-26-18(15)19(28)27-9-7-12(8-10-27)14-3-1-2-4-16(14)20(22,23)24/h1-6,11-12H,7-10H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM249544
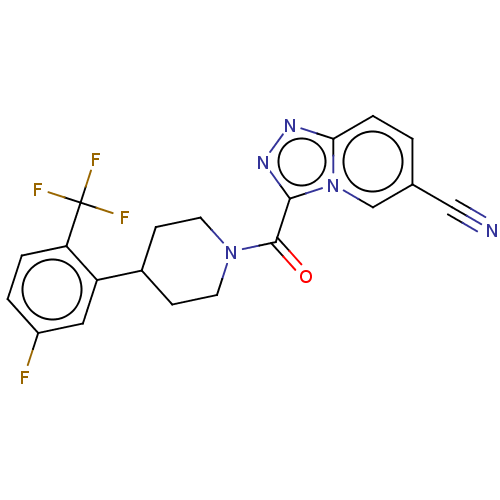
(US10072016, Compound 142 | US10407433, Compound 14...)Show SMILES Fc1ccc(c(c1)C1CCN(CC1)C(=O)c1nnc2ccc(cn12)C#N)C(F)(F)F Show InChI InChI=1S/C20H15F4N5O/c21-14-2-3-16(20(22,23)24)15(9-14)13-5-7-28(8-6-13)19(30)18-27-26-17-4-1-12(10-25)11-29(17)18/h1-4,9,11,13H,5-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501936
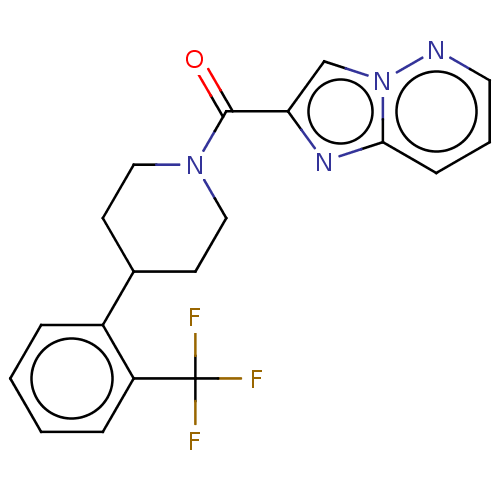
(CHEMBL4435017)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1cn2ncccc2n1 Show InChI InChI=1S/C19H17F3N4O/c20-19(21,22)15-5-2-1-4-14(15)13-7-10-25(11-8-13)18(27)16-12-26-17(24-16)6-3-9-23-26/h1-6,9,12-13H,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501900

(CHEMBL4463039)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1nnc2ccc(cn12)C#N Show InChI InChI=1S/C20H16F3N5O/c21-20(22,23)16-4-2-1-3-15(16)14-7-9-27(10-8-14)19(29)18-26-25-17-6-5-13(11-24)12-28(17)18/h1-6,12,14H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501898
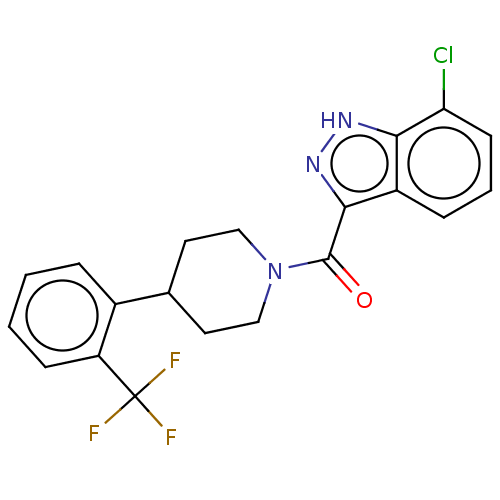
(CHEMBL4588529)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1n[nH]c2c(Cl)cccc12 Show InChI InChI=1S/C20H17ClF3N3O/c21-16-7-3-5-14-17(16)25-26-18(14)19(28)27-10-8-12(9-11-27)13-4-1-2-6-15(13)20(22,23)24/h1-7,12H,8-11H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM249466

(US10072016, Compound 65 | US10407433, Compound 65 ...)Show SMILES Fc1cccc(C2CCN(CC2)C(=O)c2nnc3ccc(cn23)C#N)c1C(F)(F)F Show InChI InChI=1S/C20H15F4N5O/c21-15-3-1-2-14(17(15)20(22,23)24)13-6-8-28(9-7-13)19(30)18-27-26-16-5-4-12(10-25)11-29(16)18/h1-5,11,13H,6-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM249559
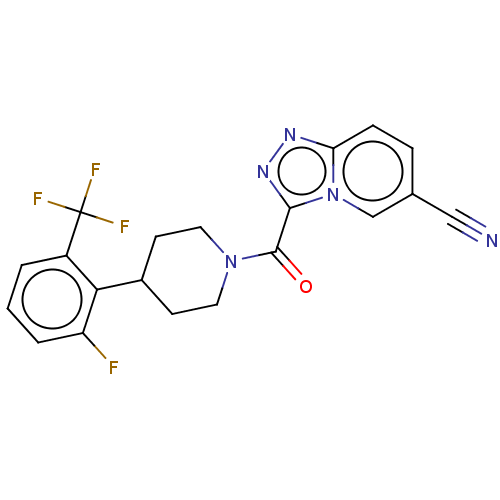
(US10072016, Compound 157 | US10407433, Compound 15...)Show SMILES Fc1cccc(c1C1CCN(CC1)C(=O)c1nnc2ccc(cn12)C#N)C(F)(F)F Show InChI InChI=1S/C20H15F4N5O/c21-15-3-1-2-14(20(22,23)24)17(15)13-6-8-28(9-7-13)19(30)18-27-26-16-5-4-12(10-25)11-29(16)18/h1-5,11,13H,6-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501896
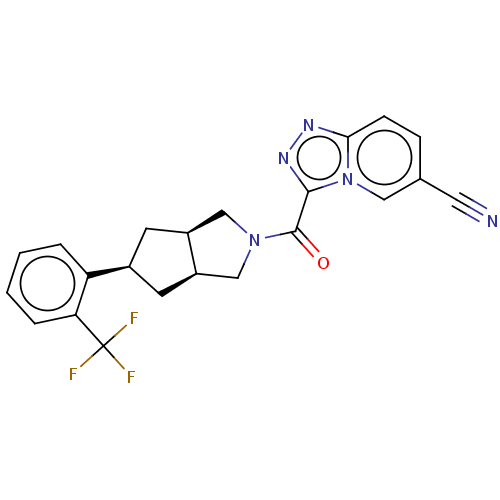
(CHEMBL4569872)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(cn12)C#N)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H18F3N5O/c23-22(24,25)18-4-2-1-3-17(18)14-7-15-11-29(12-16(15)8-14)21(31)20-28-27-19-6-5-13(9-26)10-30(19)20/h1-6,10,14-16H,7-8,11-12H2/t14-,15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501913

(CHEMBL4525563)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)c1nnc2ccc(OC)cn12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N4O2/c1-31-16-6-7-19-26-27-20(29(19)12-16)21(30)28-10-14-8-13(9-15(14)11-28)17-4-2-3-5-18(17)22(23,24)25/h2-7,12-15H,8-11H2,1H3/t13-,14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501907
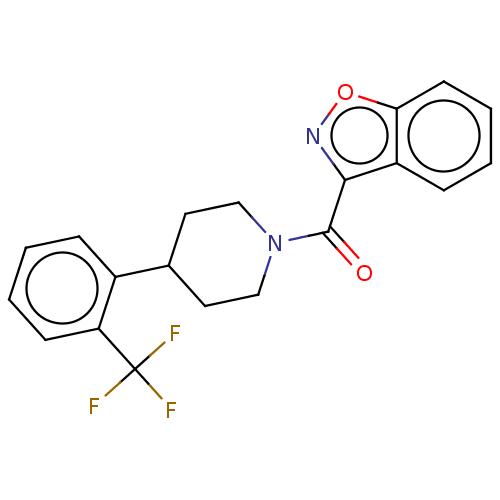
(CHEMBL4591437)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1noc2ccccc12 Show InChI InChI=1S/C20H17F3N2O2/c21-20(22,23)16-7-3-1-5-14(16)13-9-11-25(12-10-13)19(26)18-15-6-2-4-8-17(15)27-24-18/h1-8,13H,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501901
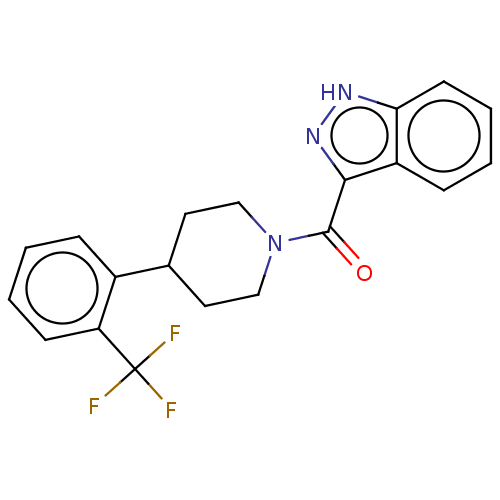
(CHEMBL4539552)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)c1n[nH]c2ccccc12 Show InChI InChI=1S/C20H18F3N3O/c21-20(22,23)16-7-3-1-5-14(16)13-9-11-26(12-10-13)19(27)18-15-6-2-4-8-17(15)24-25-18/h1-8,13H,9-12H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50501922
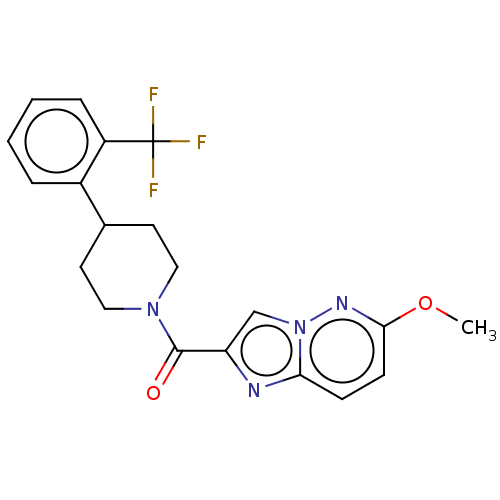
(CHEMBL4476117)Show SMILES COc1ccc2nc(cn2n1)C(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C20H19F3N4O2/c1-29-18-7-6-17-24-16(12-27(17)25-18)19(28)26-10-8-13(9-11-26)14-4-2-3-5-15(14)20(21,22)23/h2-7,12-13H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Competitive displacement of 3[H]retinol from biotinylated RBP4 (unknown origin) by surface plasmon resonance analysis |
J Med Chem 62: 5470-5500 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00352
BindingDB Entry DOI: 10.7270/Q290272Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data