

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255582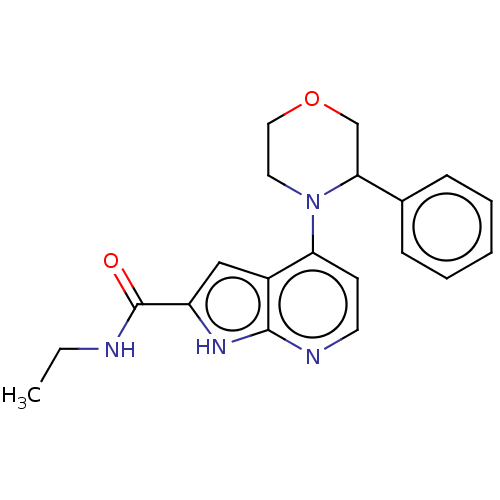 (CHEMBL4078783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255603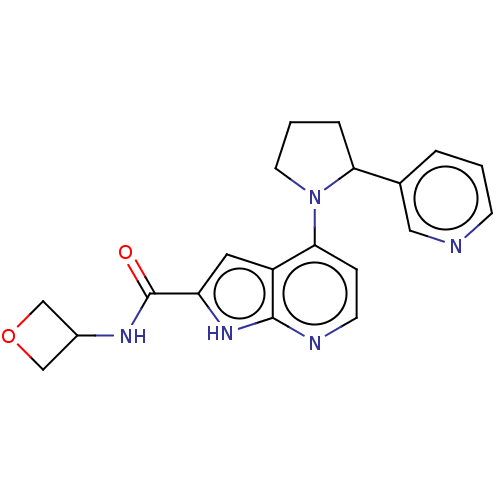 (CHEMBL4083992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255566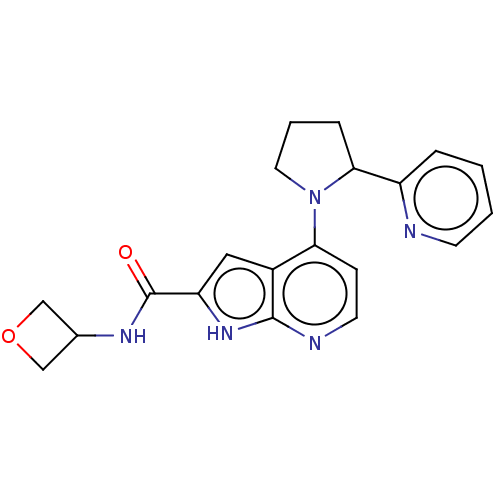 (CHEMBL4094252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255598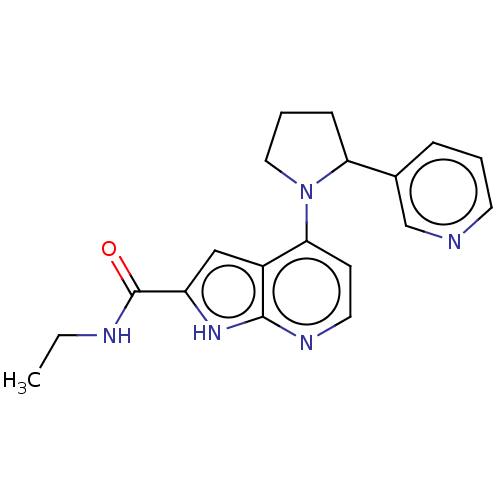 (CHEMBL4064004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255568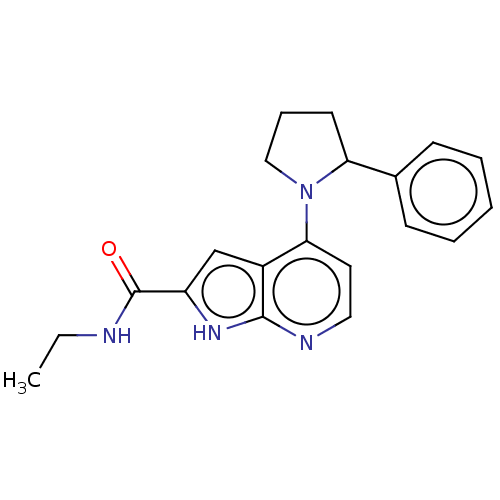 (CHEMBL4091768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255621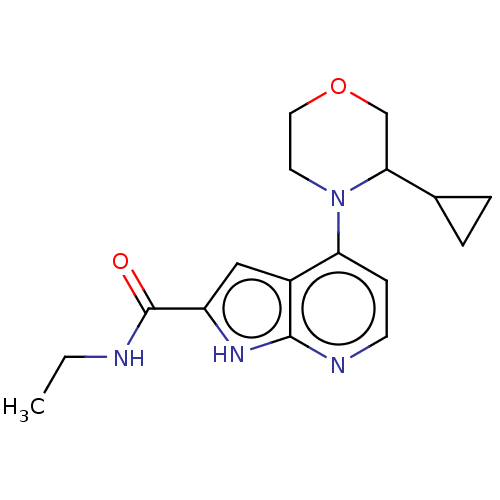 (CHEMBL4070624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255622 (CHEMBL4067896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255624 (CHEMBL4101983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255581 (CHEMBL4073623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583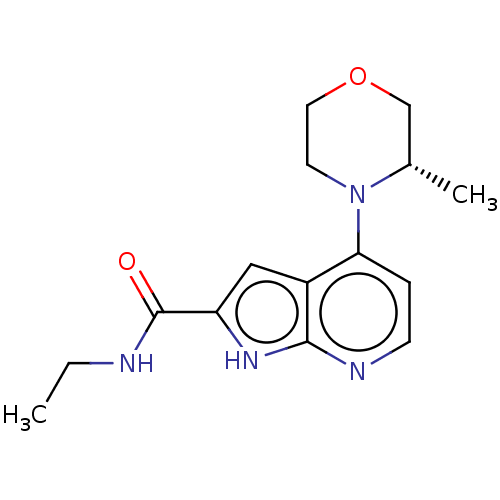 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528011 (US11179399, Example 44_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255623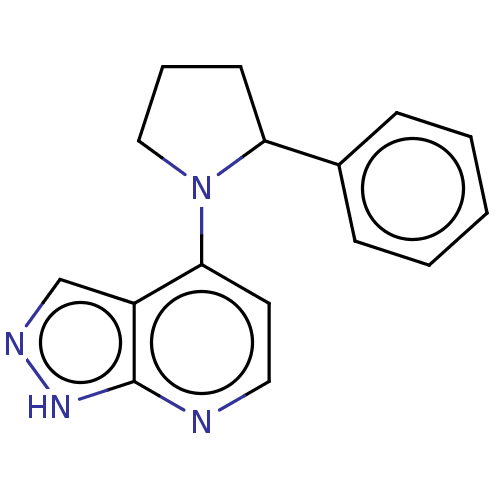 (CHEMBL4072758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50172920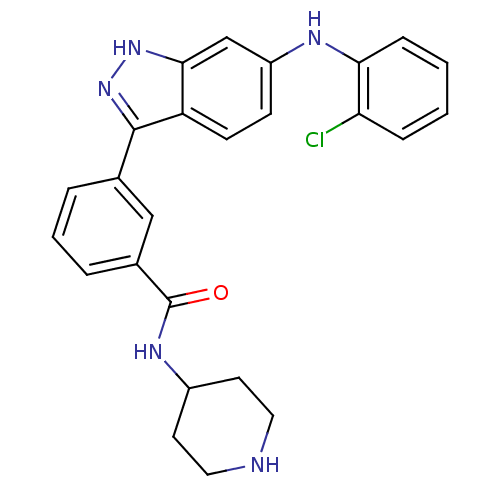 (3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against c-Jun N-terminal kinase 3 | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50211306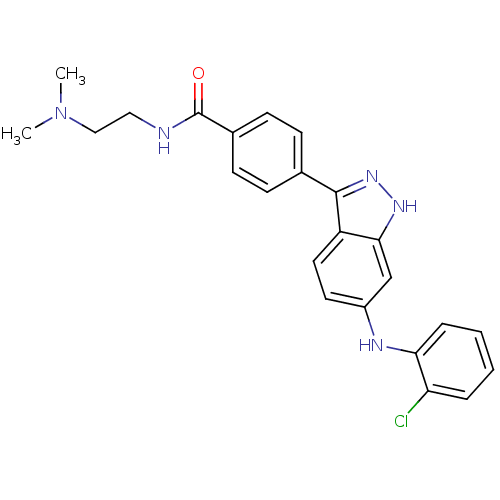 (4-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against c-Jun N-terminal kinase 3 | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255585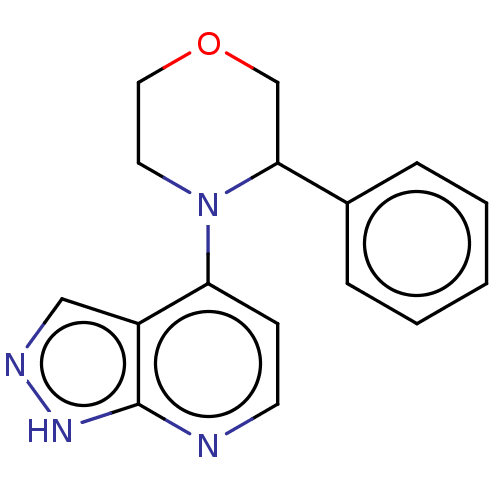 (CHEMBL4094381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527982 (US11179399, Example 25_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528009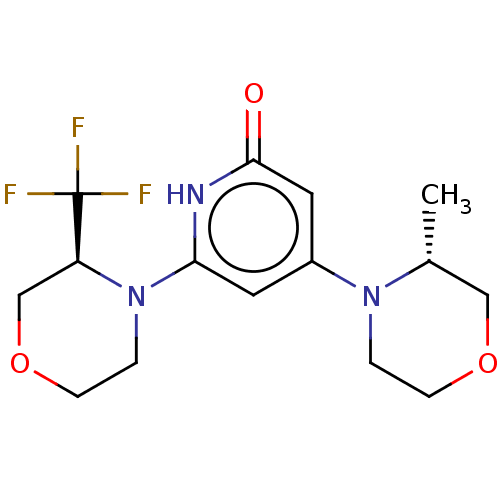 (US11179399, Example 43_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528013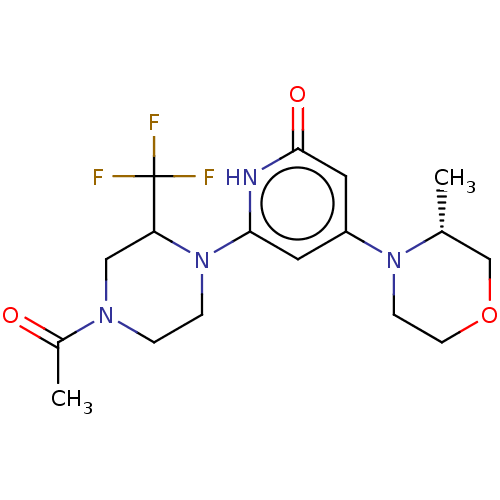 (6-[4-acetyl-2-(trifluoromethyl)piperazin-1-yl]-4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50178827 ((+)-N-(4-(2-(4-fluorophenylamino)pyridin-4-yl)pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibition of JNK3 | Bioorg Med Chem Lett 16: 1397-401 (2006) Article DOI: 10.1016/j.bmcl.2005.11.039 BindingDB Entry DOI: 10.7270/Q2KP81R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50178827 ((+)-N-(4-(2-(4-fluorophenylamino)pyridin-4-yl)pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibition of JNK3 | Bioorg Med Chem Lett 16: 1397-401 (2006) Article DOI: 10.1016/j.bmcl.2005.11.039 BindingDB Entry DOI: 10.7270/Q2KP81R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50172919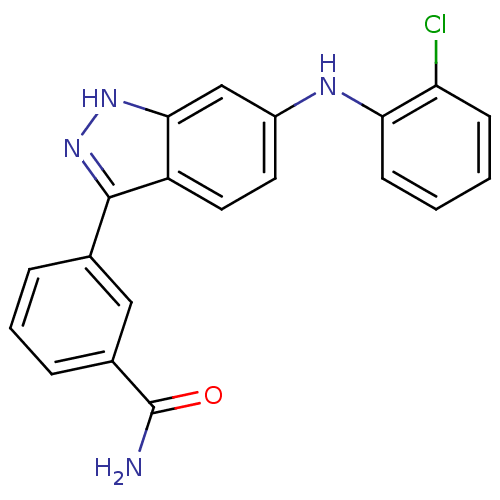 (3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50172919 (3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against c-Jun N-terminal kinase 3 | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50172921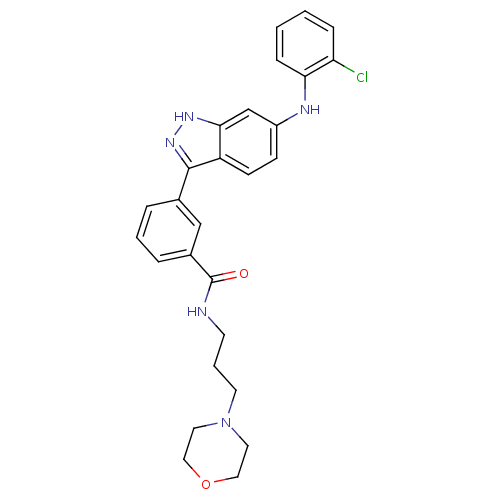 (3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-N-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against c-Jun N-terminal kinase 3 | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50172921 (3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-N-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527981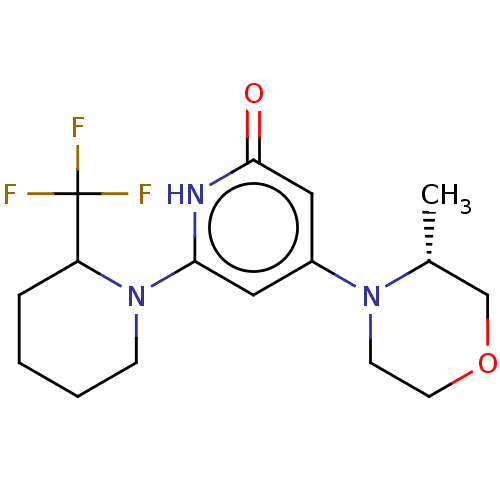 ((R) and (S) 4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527937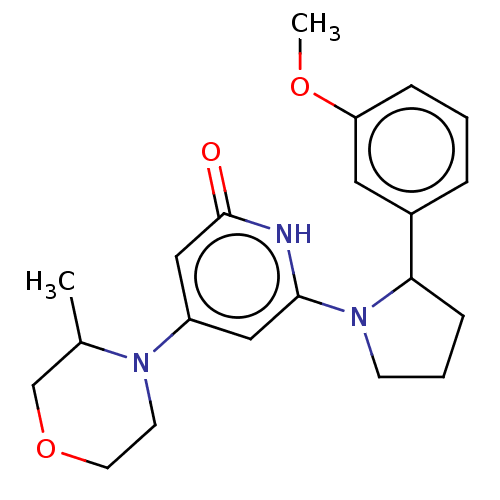 (6-[2-(3-methoxyphenyl)pyrrolidin-1-yl]-4-(3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50172920 (3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibitory concentration against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 15: 5095-9 (2005) Article DOI: 10.1016/j.bmcl.2005.06.083 BindingDB Entry DOI: 10.7270/Q2JW8DFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532962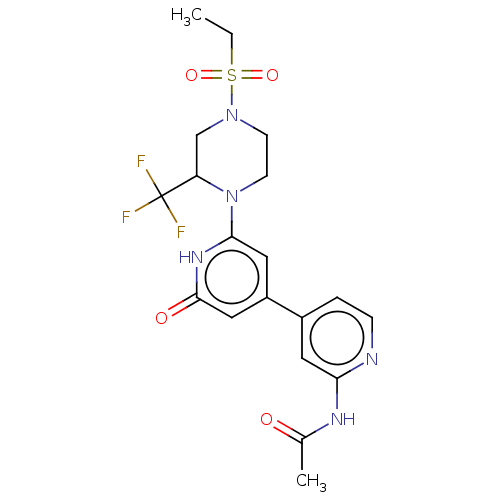 (N-[4-[2-[4-ethylsulfonyl-2-(trifluoromethyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26113H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532963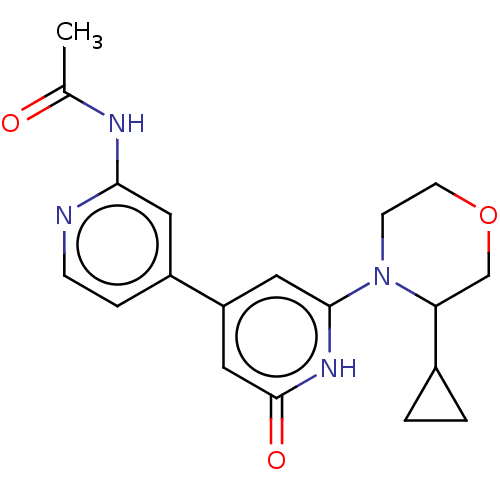 (N-[4-[2-(3-cyclopropylmorpholin-4-yl)-6-oxo-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26113H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532964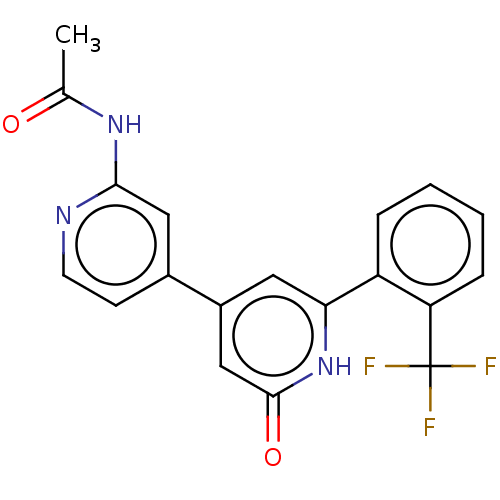 (N-[4-[2-oxo-6-[2-(trifluoromethyl)phenyl]-1H-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26113H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532965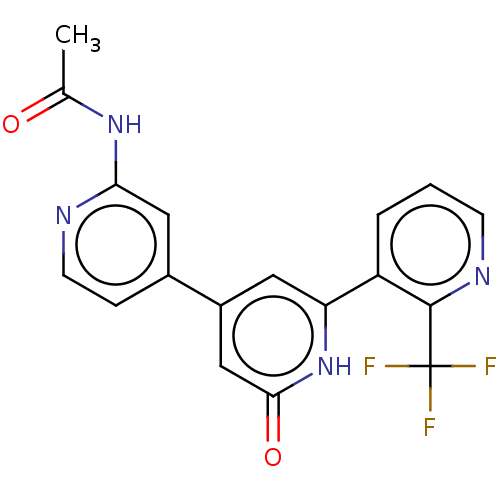 (N-[4-[2-oxo-6-[2-(trifluoromethyl)-3-pyridyl]-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26113H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50178843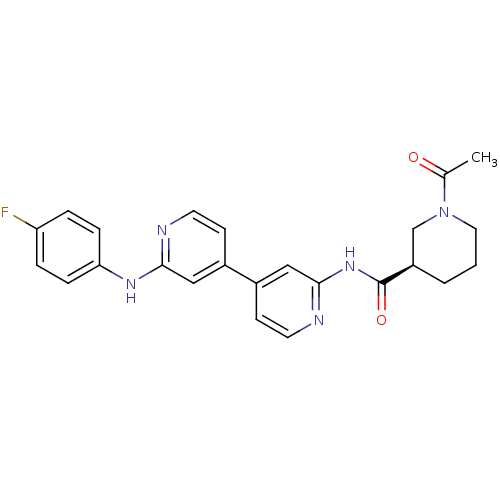 ((3R)-1-acetyl-N-(4-(2-(4-fluorophenylamino)pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Inhibition of JNK3 | Bioorg Med Chem Lett 16: 1397-401 (2006) Article DOI: 10.1016/j.bmcl.2005.11.039 BindingDB Entry DOI: 10.7270/Q2KP81R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255584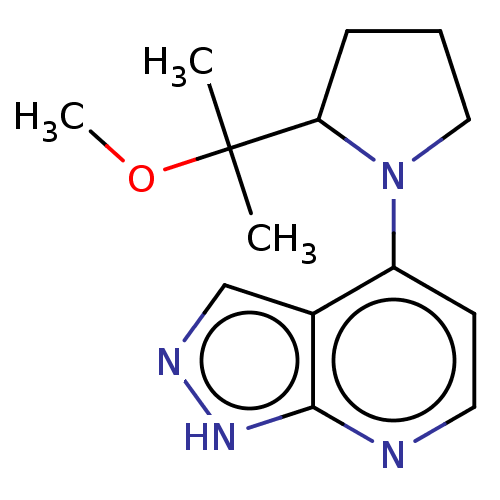 (CHEMBL4089106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255615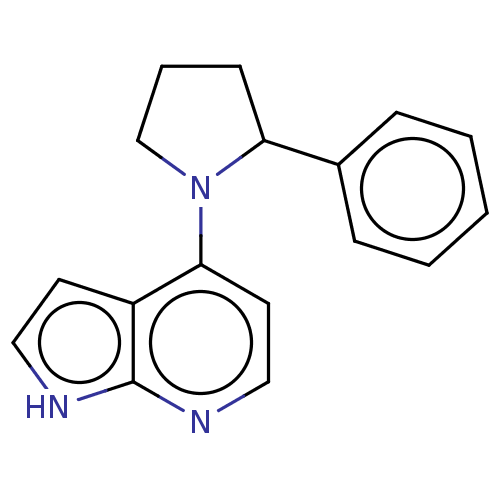 (CHEMBL4061204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532262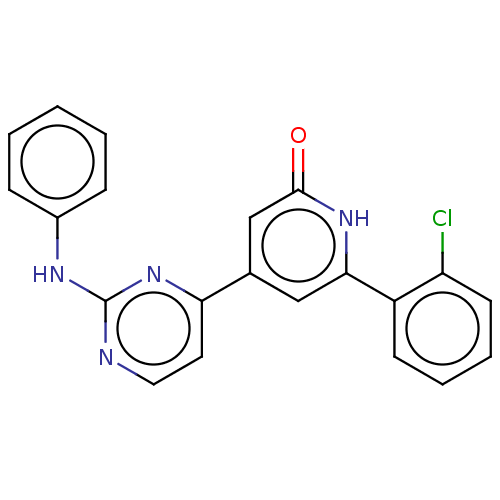 (4-(2-anilinopyrimidin-4-yl)-6-(2-chlorophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532263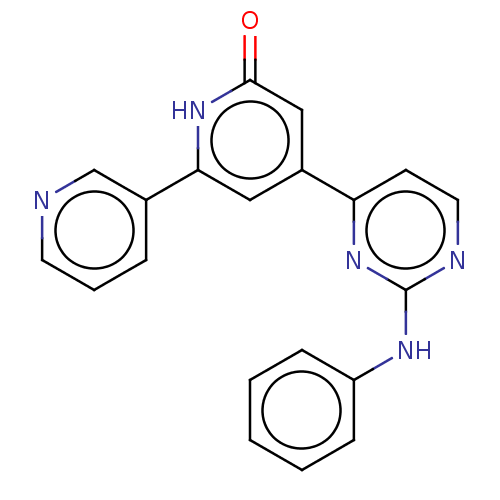 (4-(2-Anilinopyrimidin-4-yl)-6-(3-pyridyl)-1H-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532265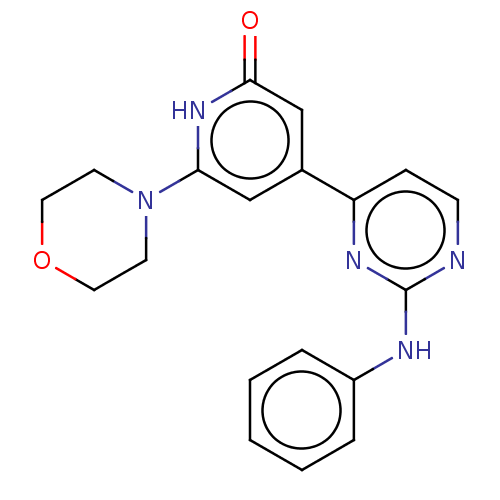 (4-(2-anilinopyrimidin-4-yl)-6-morpholino-1H-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532266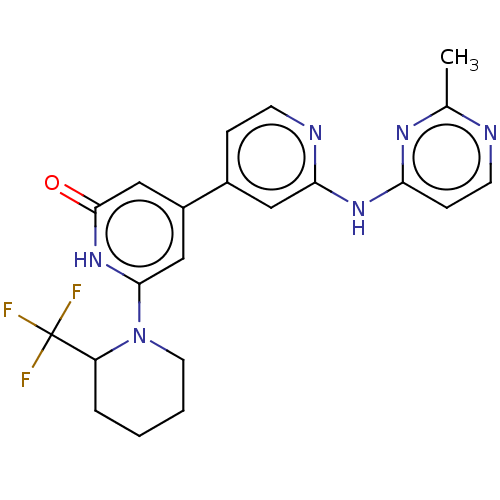 (4-[2-[(2-Methylpyrimidin-4-yl)amino]-4-pyridyl]-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532267 (4-(2-Anilinopyrimidin-4-yl)-6-[2-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532268 (4-[2-[(2-Methylpyrimidin-4-yl)amino]-4-pyridyl]-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532269 (4-(2-Anilinopyrimidin-4-yl)-6-[3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532270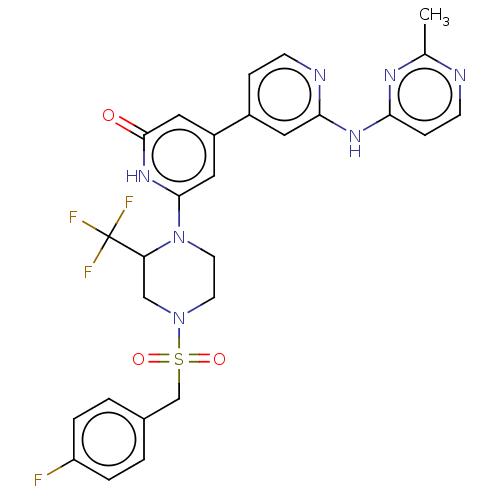 (6-[4-[(4-Fluorophenyl)methylsulfonyl]-2-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532271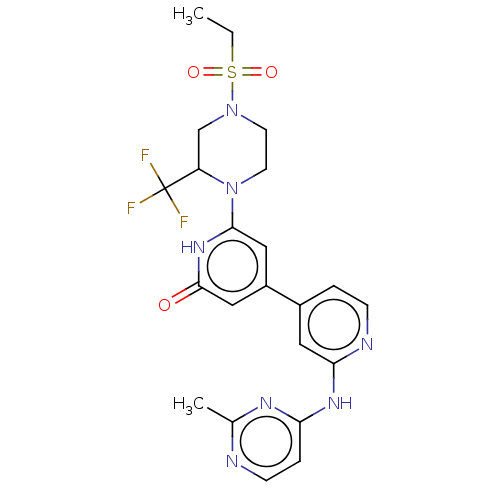 (6-[4-Ethylsulfonyl-2-(trifluoromethyl)piperazin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532272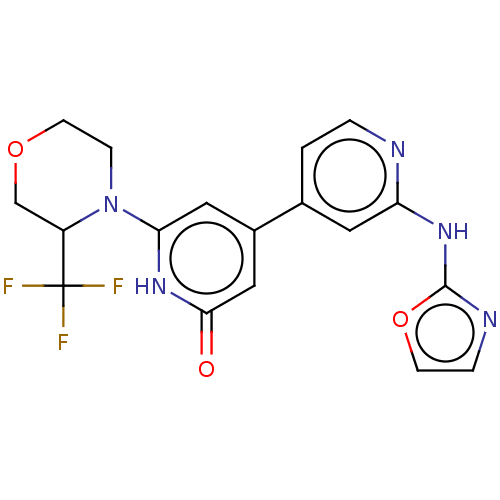 (4-[2-(Oxazol-2-ylamino)-4-pyridyl]-6-[3-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532274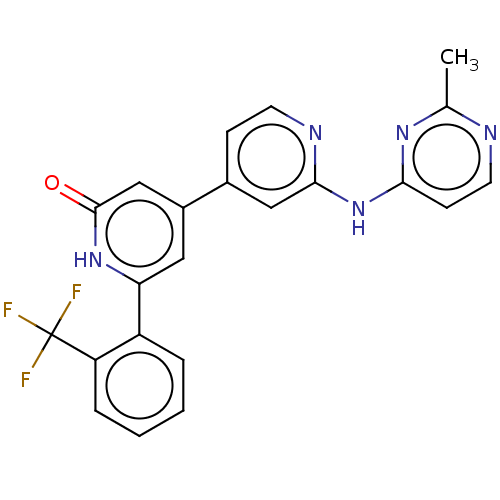 (4-[2-[(2-Methylpyrimidin-4-yl)amino]-4-pyridyl]-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532277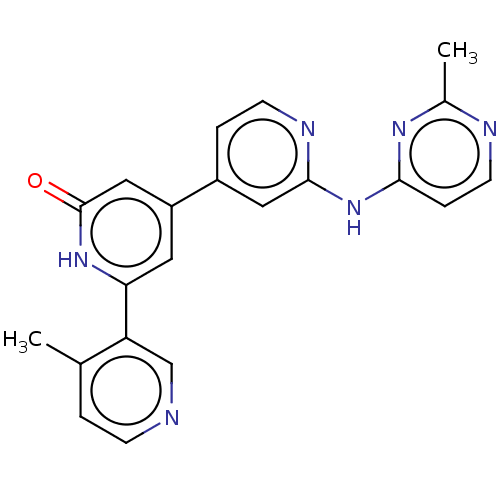 (6-(4-Methyl-3-pyridyl)-4-[2-[(2-methylpyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532278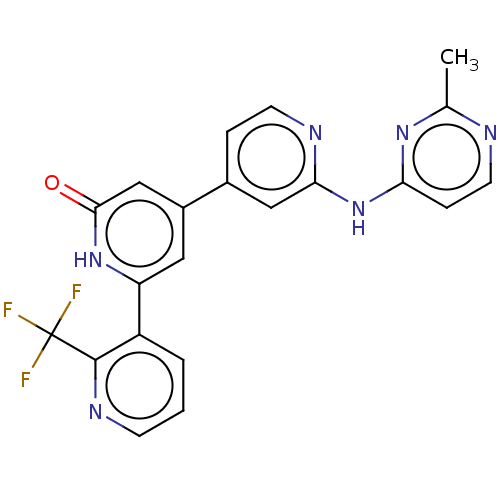 (4-[2-[(2-Methylpyrimidin-4-yl)amino]-4-pyridyl]-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532279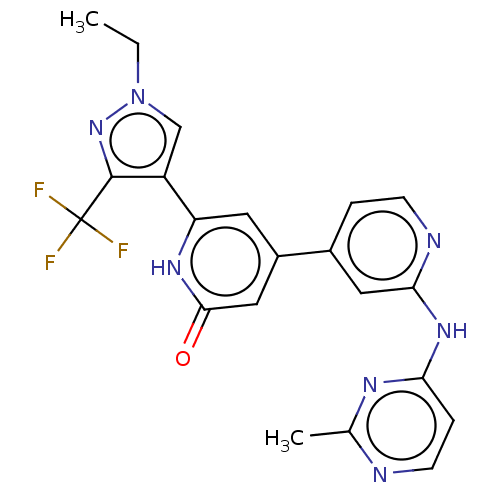 (6-[1-Ethyl-3-(trifluoromethyl)pyrazol-4-yl]-4-[2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532281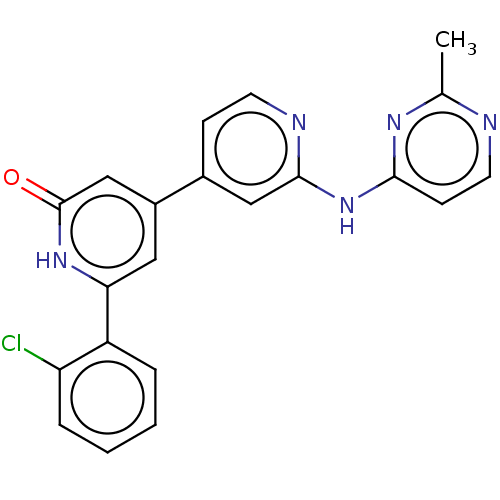 (6-(2-Chlorophenyl)-4-[2-[(2-methylpyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q28055SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM532262 (4-(2-anilinopyrimidin-4-yl)-6-(2-chlorophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 830 total ) | Next | Last >> |