 Found 44 hits with Last Name = 'wagnon' and Initial = 'j'
Found 44 hits with Last Name = 'wagnon' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(BOVINE) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903
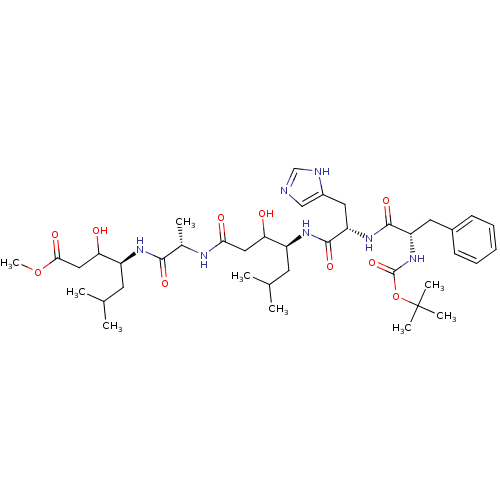
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167
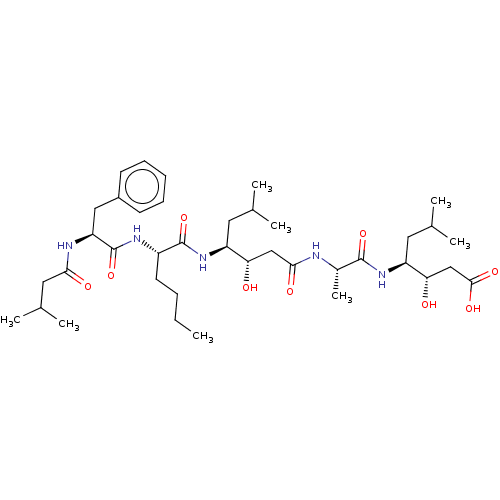
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024187
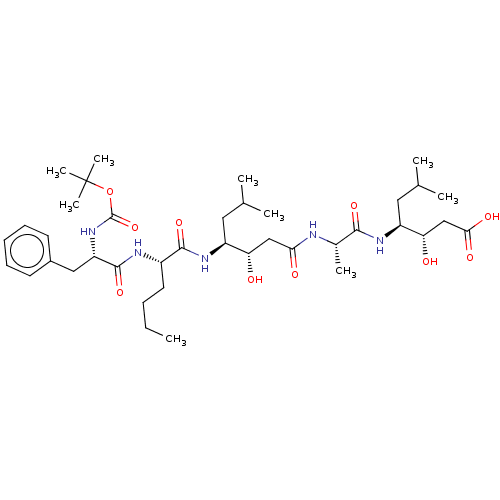
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O10/c1-10-11-17-27(41-37(52)30(20-26-15-13-12-14-16-26)44-38(53)54-39(7,8)9)36(51)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(50)42-29(19-24(4)5)32(46)22-34(48)49/h12-16,23-25,27-32,45-46H,10-11,17-22H2,1-9H3,(H,40,47)(H,41,52)(H,42,50)(H,43,51)(H,44,53)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421779

(CHEMBL308300 | CHEMBL3142338)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C1CCCC1 |r| Show InChI InChI=1S/C41H67N5O10/c1-24(2)19-29(32(47)22-34(49)42-26(5)37(51)43-30(20-25(3)4)33(48)23-35(50)55-9)44-39(53)36(28-17-13-14-18-28)46-38(52)31(21-27-15-11-10-12-16-27)45-40(54)56-41(6,7)8/h10-12,15-16,24-26,28-33,36,47-48H,13-14,17-23H2,1-9H3,(H,42,49)(H,43,51)(H,44,53)(H,45,54)(H,46,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421775

(CHEMBL3142326 | CHEMBL73556)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(42-39(52)31(41-34(47)19-25(6)7)20-27-15-12-11-13-16-27)38(51)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)43-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,41,47)(H,42,52)(H,43,50)(H,44,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50226299

(CHEMBL3142798)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O10/c1-11-15-27(41-37(51)30(20-26-16-13-12-14-17-26)44-38(52)54-39(7,8)9)36(50)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(49)42-29(19-24(4)5)32(46)22-34(48)53-10/h12-14,16-17,23-25,27-32,45-46H,11,15,18-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52)/t25-,27-,28-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421770

(CHEMBL2311099)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C39H65N5O10/c1-22(2)17-27(30(45)20-32(47)40-25(7)35(49)41-28(18-23(3)4)31(46)21-33(48)53-11)42-37(51)34(24(5)6)44-36(50)29(19-26-15-13-12-14-16-26)43-38(52)54-39(8,9)10/h12-16,22-25,27-31,34,45-46H,17-21H2,1-11H3,(H,40,47)(H,41,49)(H,42,51)(H,43,52)(H,44,50)/t25-,27-,28-,29-,30?,31?,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421783
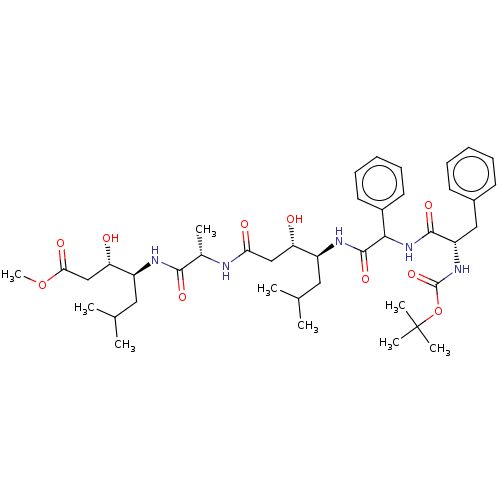
(CHEMBL3142339 | CHEMBL74979)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C42H63N5O10/c1-25(2)20-30(33(48)23-35(50)43-27(5)38(52)44-31(21-26(3)4)34(49)24-36(51)56-9)45-40(54)37(29-18-14-11-15-19-29)47-39(53)32(22-28-16-12-10-13-17-28)46-41(55)57-42(6,7)8/h10-19,25-27,30-34,37,48-49H,20-24H2,1-9H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421778
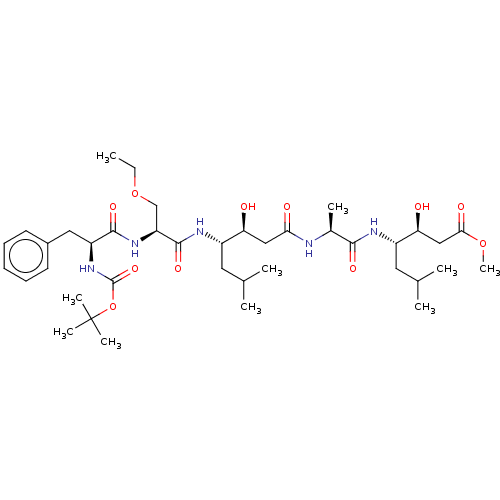
(CHEMBL3142325 | CHEMBL75458)Show SMILES CCOC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O11/c1-11-54-22-30(43-36(50)29(19-26-15-13-12-14-16-26)44-38(52)55-39(7,8)9)37(51)42-27(17-23(2)3)31(45)20-33(47)40-25(6)35(49)41-28(18-24(4)5)32(46)21-34(48)53-10/h12-16,23-25,27-32,45-46H,11,17-22H2,1-10H3,(H,40,47)(H,41,49)(H,42,51)(H,43,50)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421769
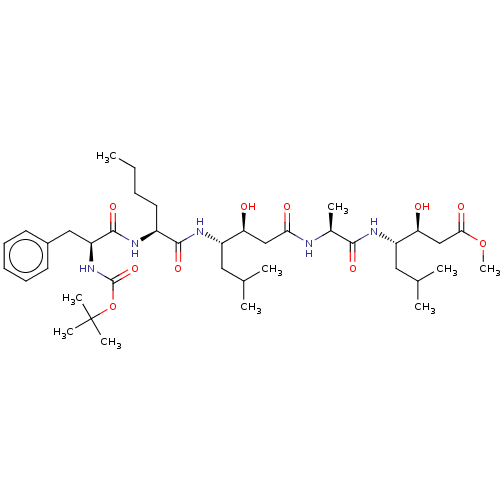
(CHEMBL3142320 | CHEMBL75210)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C40H67N5O10/c1-11-12-18-28(42-38(52)31(21-27-16-14-13-15-17-27)45-39(53)55-40(7,8)9)37(51)44-29(19-24(2)3)32(46)22-34(48)41-26(6)36(50)43-30(20-25(4)5)33(47)23-35(49)54-10/h13-17,24-26,28-33,46-47H,11-12,18-23H2,1-10H3,(H,41,48)(H,42,52)(H,43,50)(H,44,51)(H,45,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421776

(CHEMBL310597 | CHEMBL3142315)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C45H66N6O10/c1-26(2)19-33(37(52)23-39(54)47-28(5)41(56)48-34(20-27(3)4)38(53)24-40(55)60-9)49-43(58)36(22-30-25-46-32-18-14-13-17-31(30)32)50-42(57)35(21-29-15-11-10-12-16-29)51-44(59)61-45(6,7)8/h10-18,25-28,33-38,46,52-53H,19-24H2,1-9H3,(H,47,54)(H,48,56)(H,49,58)(H,50,57)(H,51,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024181
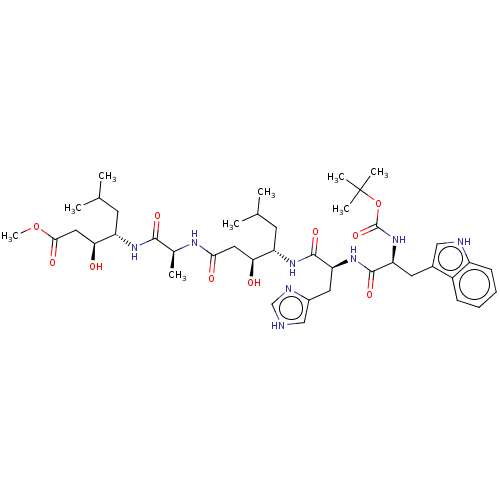
(4-(2-{4-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C42H64N8O10/c1-23(2)14-30(34(51)18-36(53)46-25(5)38(55)47-31(15-24(3)4)35(52)19-37(54)59-9)48-40(57)33(17-27-21-43-22-45-27)49-39(56)32(50-41(58)60-42(6,7)8)16-26-20-44-29-13-11-10-12-28(26)29/h10-13,20-25,30-35,44,51-52H,14-19H2,1-9H3,(H,43,45)(H,46,53)(H,47,55)(H,48,57)(H,49,56)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421767
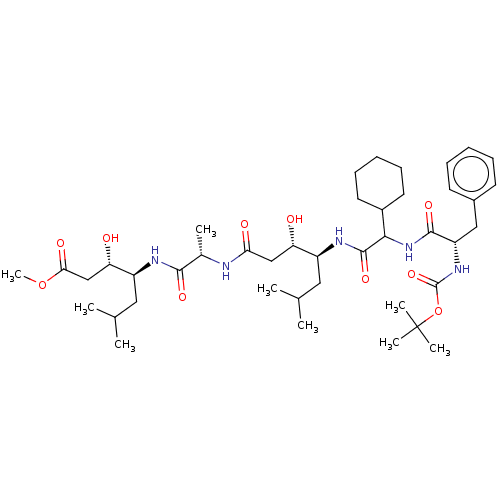
(CHEMBL312075 | CHEMBL3142324)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C1CCCCC1 |r| Show InChI InChI=1S/C42H69N5O10/c1-25(2)20-30(33(48)23-35(50)43-27(5)38(52)44-31(21-26(3)4)34(49)24-36(51)56-9)45-40(54)37(29-18-14-11-15-19-29)47-39(53)32(22-28-16-12-10-13-17-28)46-41(55)57-42(6,7)8/h10,12-13,16-17,25-27,29-34,37,48-49H,11,14-15,18-24H2,1-9H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022516

(Boc-Trp-Trp-Sta-Ala-Sta-OMe | CHEMBL3142766)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C47H67N7O10/c1-26(2)18-35(39(55)22-41(57)50-28(5)43(59)51-36(19-27(3)4)40(56)23-42(58)63-9)52-44(60)37(20-29-24-48-33-16-12-10-14-31(29)33)53-45(61)38(54-46(62)64-47(6,7)8)21-30-25-49-34-17-13-11-15-32(30)34/h10-17,24-28,35-40,48-49,55-56H,18-23H2,1-9H3,(H,50,57)(H,51,59)(H,52,60)(H,53,61)(H,54,62)/t28-,35?,36?,37-,38-,39?,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421771

(CHEMBL307703 | CHEMBL3142321)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](COCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C44H67N5O11/c1-27(2)20-32(36(50)23-38(52)45-29(5)40(54)46-33(21-28(3)4)37(51)24-39(53)58-9)47-42(56)35(26-59-25-31-18-14-11-15-19-31)48-41(55)34(22-30-16-12-10-13-17-30)49-43(57)60-44(6,7)8/h10-19,27-29,32-37,50-51H,20-26H2,1-9H3,(H,45,52)(H,46,54)(H,47,56)(H,48,55)(H,49,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421761
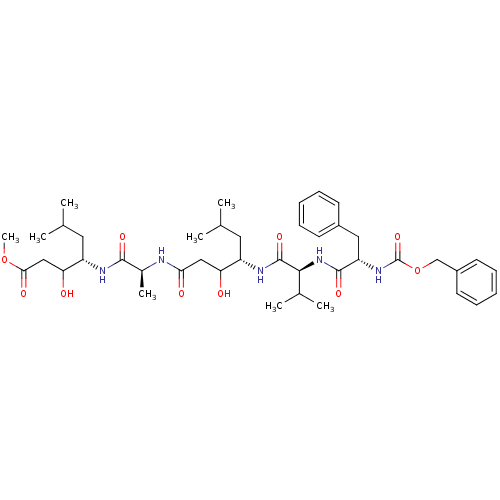
(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024174
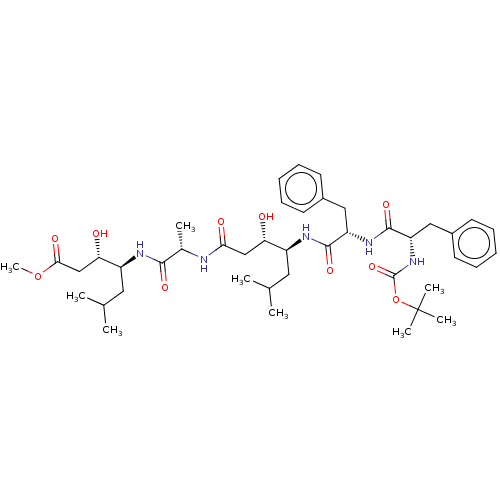
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421772
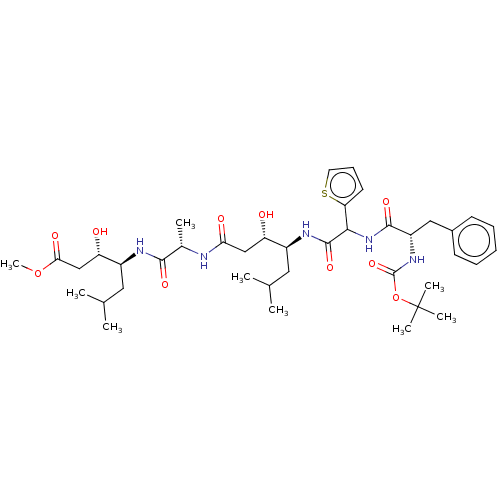
(CHEMBL3142323 | CHEMBL75470)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)c1cccs1 |r| Show InChI InChI=1S/C40H61N5O10S/c1-23(2)18-27(30(46)21-33(48)41-25(5)36(50)42-28(19-24(3)4)31(47)22-34(49)54-9)43-38(52)35(32-16-13-17-56-32)45-37(51)29(20-26-14-11-10-12-15-26)44-39(53)55-40(6,7)8/h10-17,23-25,27-31,35,46-47H,18-22H2,1-9H3,(H,41,48)(H,42,50)(H,43,52)(H,44,53)(H,45,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421768
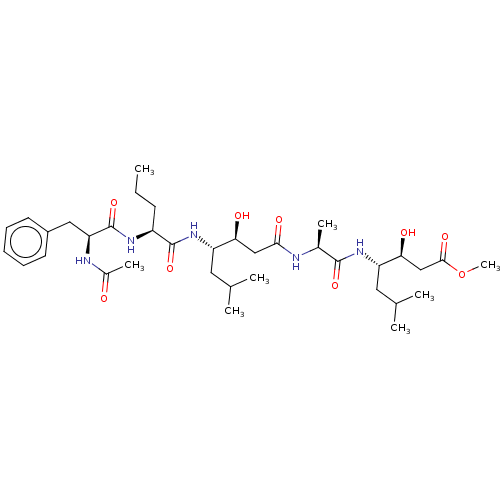
(CHEMBL3142319 | CHEMBL75987)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C36H59N5O9/c1-9-13-26(39-36(49)29(38-24(7)42)18-25-14-11-10-12-15-25)35(48)41-27(16-21(2)3)30(43)19-32(45)37-23(6)34(47)40-28(17-22(4)5)31(44)20-33(46)50-8/h10-12,14-15,21-23,26-31,43-44H,9,13,16-20H2,1-8H3,(H,37,45)(H,38,42)(H,39,49)(H,40,47)(H,41,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024195
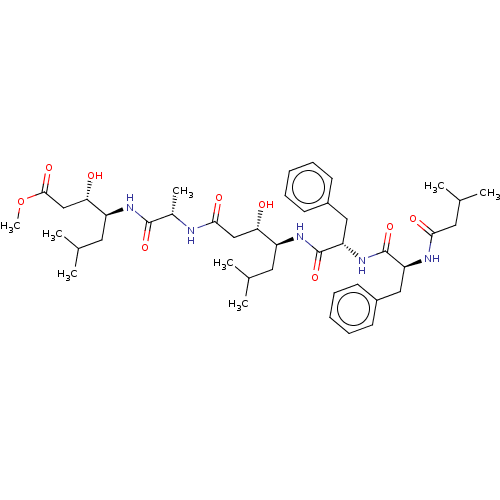
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C |r| Show InChI InChI=1S/C43H65N5O9/c1-26(2)19-32(36(49)24-39(52)44-29(7)41(54)46-33(20-27(3)4)37(50)25-40(53)57-8)47-43(56)35(23-31-17-13-10-14-18-31)48-42(55)34(45-38(51)21-28(5)6)22-30-15-11-9-12-16-30/h9-18,26-29,32-37,49-50H,19-25H2,1-8H3,(H,44,52)(H,45,51)(H,46,54)(H,47,56)(H,48,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421773
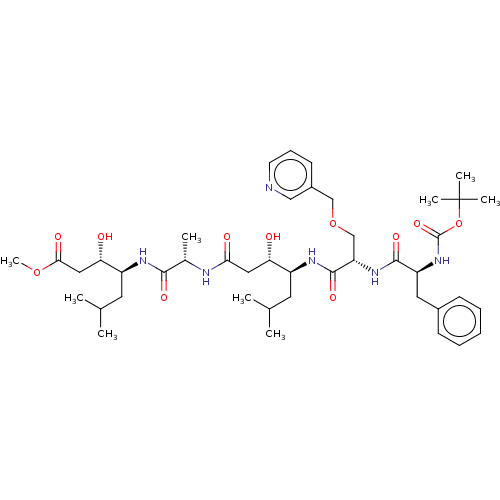
(CHEMBL306286 | CHEMBL3142330)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](COCc1cccnc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H66N6O11/c1-26(2)18-31(35(50)21-37(52)45-28(5)39(54)46-32(19-27(3)4)36(51)22-38(53)58-9)47-41(56)34(25-59-24-30-16-13-17-44-23-30)48-40(55)33(20-29-14-11-10-12-15-29)49-42(57)60-43(6,7)8/h10-17,23,26-28,31-36,50-51H,18-22,24-25H2,1-9H3,(H,45,52)(H,46,54)(H,47,56)(H,48,55)(H,49,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421777
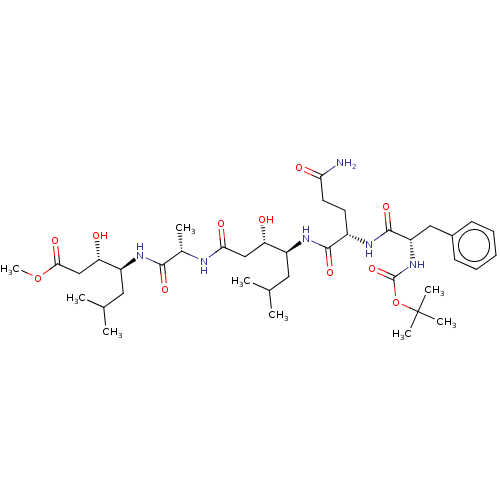
(CHEMBL3142336 | CHEMBL407937)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H64N6O11/c1-22(2)17-27(30(46)20-33(49)41-24(5)35(51)43-28(18-23(3)4)31(47)21-34(50)55-9)44-36(52)26(15-16-32(40)48)42-37(53)29(19-25-13-11-10-12-14-25)45-38(54)56-39(6,7)8/h10-14,22-24,26-31,46-47H,15-21H2,1-9H3,(H2,40,48)(H,41,49)(H,42,53)(H,43,51)(H,44,52)(H,45,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421766
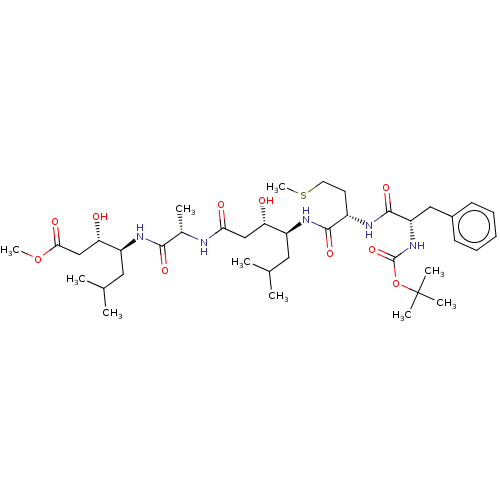
(CHEMBL3142335 | CHEMBL74682)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H65N5O10S/c1-23(2)18-28(31(45)21-33(47)40-25(5)35(49)42-29(19-24(3)4)32(46)22-34(48)53-9)43-36(50)27(16-17-55-10)41-37(51)30(20-26-14-12-11-13-15-26)44-38(52)54-39(6,7)8/h11-15,23-25,27-32,45-46H,16-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421765
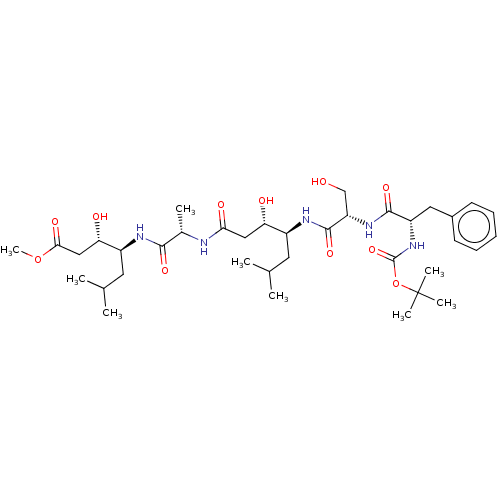
(CHEMBL3142334 | CHEMBL73705)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C37H61N5O11/c1-21(2)15-25(29(44)18-31(46)38-23(5)33(48)39-26(16-22(3)4)30(45)19-32(47)52-9)40-35(50)28(20-43)41-34(49)27(17-24-13-11-10-12-14-24)42-36(51)53-37(6,7)8/h10-14,21-23,25-30,43-45H,15-20H2,1-9H3,(H,38,46)(H,39,48)(H,40,50)(H,41,49)(H,42,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421784
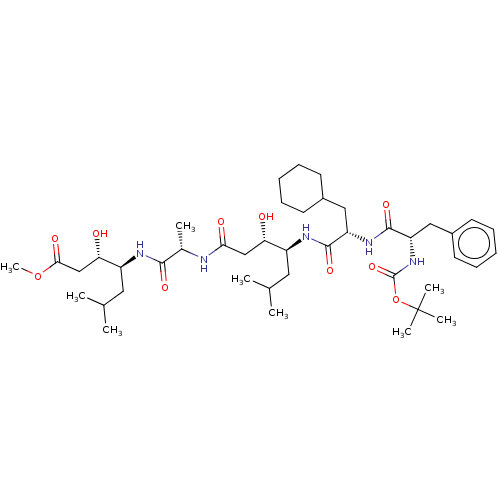
(CHEMBL3142337 | CHEMBL74470)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H71N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h11,14-15,18-19,26-29,31-36,49-50H,10,12-13,16-17,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50226300
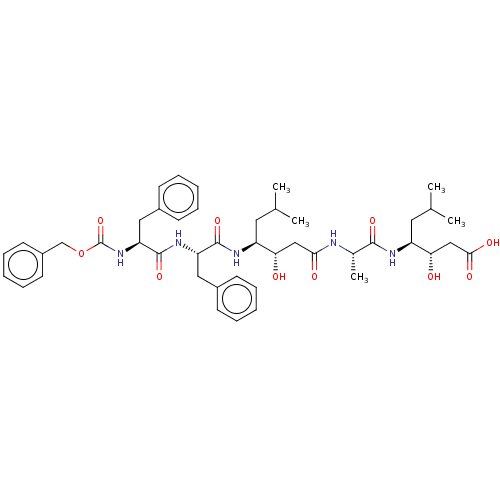
(CHEMBL3142776)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C45H61N5O10/c1-28(2)21-34(38(51)25-40(53)46-30(5)42(56)47-35(22-29(3)4)39(52)26-41(54)55)48-43(57)36(23-31-15-9-6-10-16-31)49-44(58)37(24-32-17-11-7-12-18-32)50-45(59)60-27-33-19-13-8-14-20-33/h6-20,28-30,34-39,51-52H,21-27H2,1-5H3,(H,46,53)(H,47,56)(H,48,57)(H,49,58)(H,50,59)(H,54,55)/t30-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421782
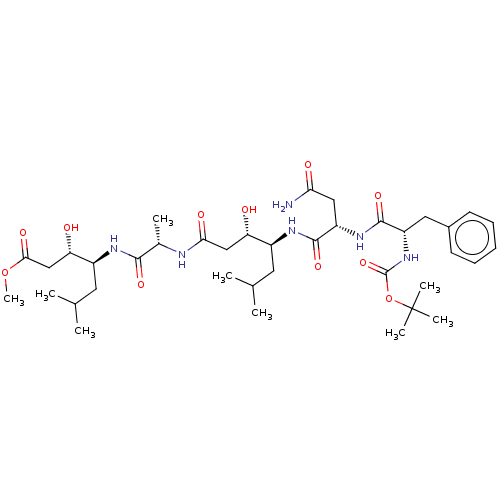
(CHEMBL3142328 | CHEMBL74904)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C38H62N6O11/c1-21(2)15-25(29(45)19-32(48)40-23(5)34(50)41-26(16-22(3)4)30(46)20-33(49)54-9)42-36(52)28(18-31(39)47)43-35(51)27(17-24-13-11-10-12-14-24)44-37(53)55-38(6,7)8/h10-14,21-23,25-30,45-46H,15-20H2,1-9H3,(H2,39,47)(H,40,48)(H,41,50)(H,42,52)(H,43,51)(H,44,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024192
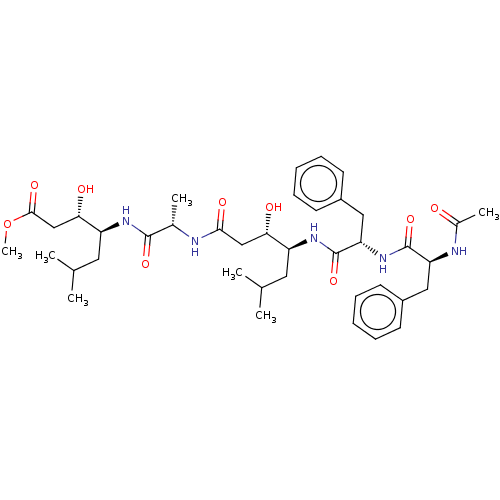
(4-(2-{4-[2-(2-Acetylamino-3-phenyl-propionylamino)...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H59N5O9/c1-24(2)18-30(34(47)22-36(49)41-26(5)38(51)43-31(19-25(3)4)35(48)23-37(50)54-7)44-40(53)33(21-29-16-12-9-13-17-29)45-39(52)32(42-27(6)46)20-28-14-10-8-11-15-28/h8-17,24-26,30-35,47-48H,18-23H2,1-7H3,(H,41,49)(H,42,46)(H,43,51)(H,44,53)(H,45,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024169
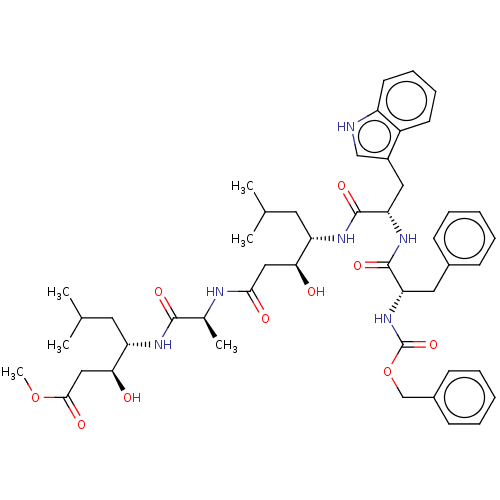
(4-(2-{4-[2-(2-Benzyloxycarbonylamino-3-phenyl-prop...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C48H64N6O10/c1-29(2)21-37(41(55)25-43(57)50-31(5)45(59)51-38(22-30(3)4)42(56)26-44(58)63-6)52-47(61)40(24-34-27-49-36-20-14-13-19-35(34)36)53-46(60)39(23-32-15-9-7-10-16-32)54-48(62)64-28-33-17-11-8-12-18-33/h7-20,27,29-31,37-42,49,55-56H,21-26,28H2,1-6H3,(H,50,57)(H,51,59)(H,52,61)(H,53,60)(H,54,62) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421780
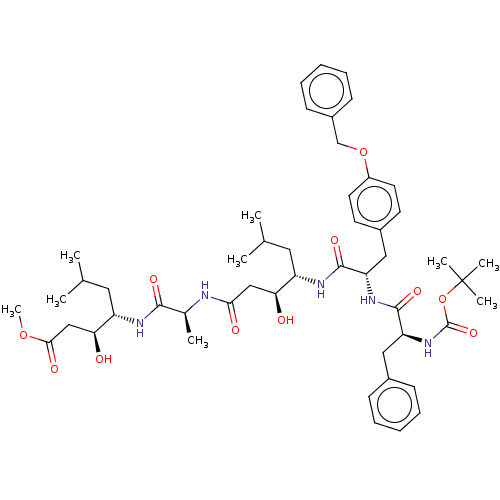
(CHEMBL3142327 | CHEMBL75557)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C50H71N5O11/c1-31(2)24-38(42(56)28-44(58)51-33(5)46(60)52-39(25-32(3)4)43(57)29-45(59)64-9)53-47(61)40(27-35-20-22-37(23-21-35)65-30-36-18-14-11-15-19-36)54-48(62)41(26-34-16-12-10-13-17-34)55-49(63)66-50(6,7)8/h10-23,31-33,38-43,56-57H,24-30H2,1-9H3,(H,51,58)(H,52,60)(H,53,61)(H,54,62)(H,55,63) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421781
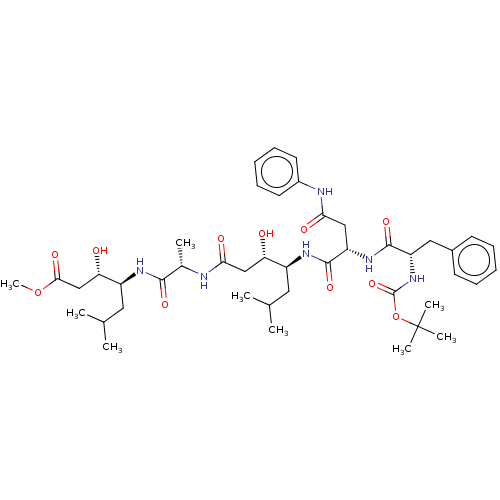
(CHEMBL306285 | CHEMBL3142322)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)Nc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C44H66N6O11/c1-26(2)20-31(35(51)24-38(54)45-28(5)40(56)47-32(21-27(3)4)36(52)25-39(55)60-9)48-42(58)34(23-37(53)46-30-18-14-11-15-19-30)49-41(57)33(22-29-16-12-10-13-17-29)50-43(59)61-44(6,7)8/h10-19,26-28,31-36,51-52H,20-25H2,1-9H3,(H,45,54)(H,46,53)(H,47,56)(H,48,58)(H,49,57)(H,50,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024166
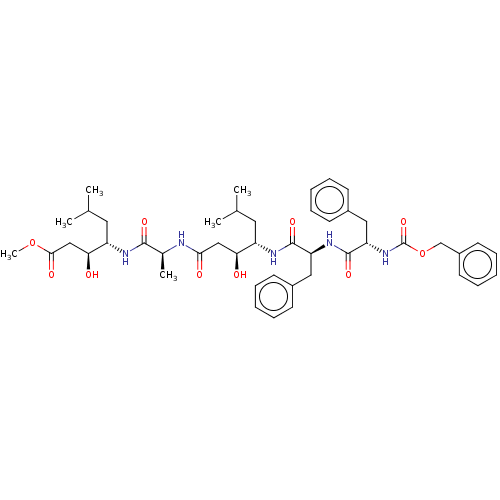
(4-(2-{4-[2-(2-Benzyloxycarbonylamino-3-phenyl-prop...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C46H63N5O10/c1-29(2)22-35(39(52)26-41(54)47-31(5)43(56)48-36(23-30(3)4)40(53)27-42(55)60-6)49-44(57)37(24-32-16-10-7-11-17-32)50-45(58)38(25-33-18-12-8-13-19-33)51-46(59)61-28-34-20-14-9-15-21-34/h7-21,29-31,35-40,52-53H,22-28H2,1-6H3,(H,47,54)(H,48,56)(H,49,57)(H,50,58)(H,51,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421774
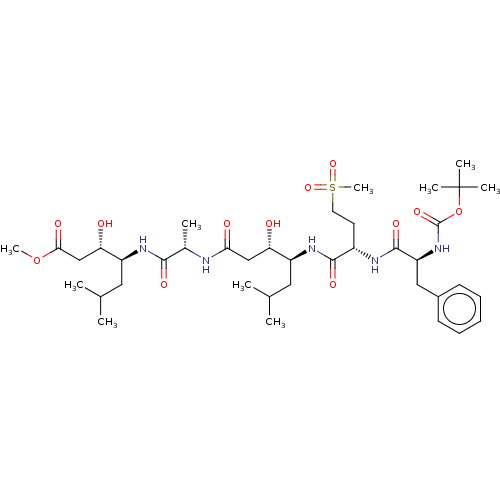
(CHEMBL3142333 | CHEMBL72931)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCS(C)(=O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H65N5O12S/c1-23(2)18-28(31(45)21-33(47)40-25(5)35(49)42-29(19-24(3)4)32(46)22-34(48)55-9)43-36(50)27(16-17-57(10,53)54)41-37(51)30(20-26-14-12-11-13-15-26)44-38(52)56-39(6,7)8/h11-15,23-25,27-32,45-46H,16-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data