 Found 59 hits with Last Name = 'wang' and Initial = 'lx'
Found 59 hits with Last Name = 'wang' and Initial = 'lx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465346

(CHEMBL4286991)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)C |r| Show InChI InChI=1S/C20H28O3/c1-13(2)14-5-7-16-15(11-14)6-8-17-19(16,3)9-4-10-20(17,12-21)18(22)23/h5,7,11,13,17,21H,4,6,8-10,12H2,1-3H3,(H,22,23)/t17-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF (unknown origin) by Lanthascreen assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055889

(CHEMBL3317913)Show SMILES O=C(N1CCN(CC1)C(=O)c1cccs1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C23H20N4O3S2/c28-21-18-5-2-1-4-17(18)19(24-25-21)13-16-12-15(14-32-16)22(29)26-7-9-27(10-8-26)23(30)20-6-3-11-31-20/h1-6,11-12,14H,7-10,13H2,(H,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465342
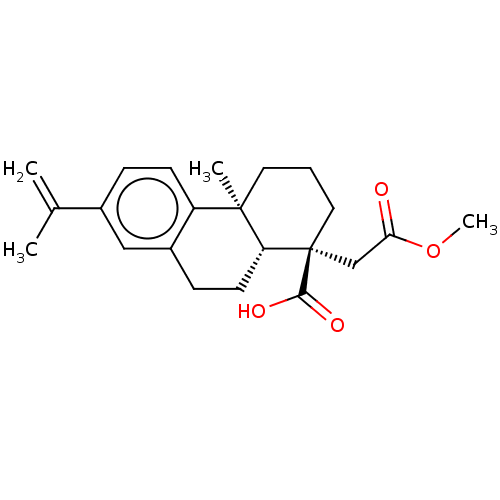
(CHEMBL4293812)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C22H28O4/c1-14(2)15-6-8-17-16(12-15)7-9-18-21(17,3)10-5-11-22(18,20(24)25)13-19(23)26-4/h6,8,12,18H,1,5,7,9-11,13H2,2-4H3,(H,24,25)/t18-,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) by Lanthascreen assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by mobility shift... |
Eur J Med Chem 141: 538-551 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.027
BindingDB Entry DOI: 10.7270/Q2V98BMQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465348

(CHEMBL4292650)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)C |r| Show InChI InChI=1S/C22H30O4/c1-14(2)15-6-8-17-16(12-15)7-9-18-21(17,3)10-5-11-22(18,20(24)25)13-19(23)26-4/h6,8,12,14,18H,5,7,9-11,13H2,1-4H3,(H,24,25)/t18-,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of GSK3b (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50143600

(CHEBI:29571 | Dehydroabietic Acid)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(C)C(O)=O)C(C)C |r| Show InChI InChI=1S/C20H28O2/c1-13(2)14-6-8-16-15(12-14)7-9-17-19(16,3)10-5-11-20(17,4)18(21)22/h6,8,12-13,17H,5,7,9-11H2,1-4H3,(H,21,22)/t17-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465345
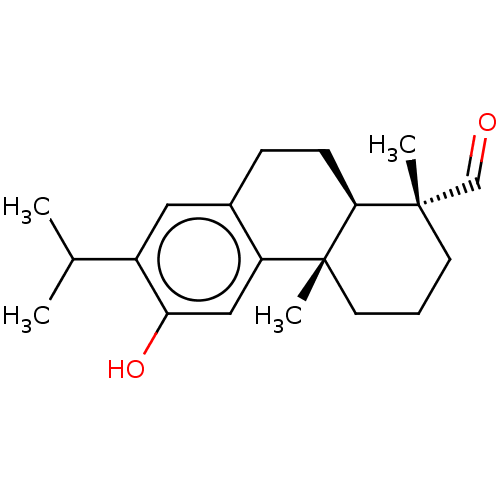
(18-Oxoferruginol | acs.jmedchem.1c00409_ST.768)Show SMILES [H][C@@]12CCc3cc(C(C)C)c(O)cc3[C@@]1(C)CCC[C@@]2(C)C=O |r| Show InChI InChI=1S/C20H28O2/c1-13(2)15-10-14-6-7-18-19(3,12-21)8-5-9-20(18,4)16(14)11-17(15)22/h10-13,18,22H,5-9H2,1-4H3/t18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055890

(CHEMBL3317912)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccc2OCOc2c1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H22N4O5S/c31-24-20-4-2-1-3-19(20)21(27-28-24)13-18-11-17(14-36-18)26(33)30-9-7-29(8-10-30)25(32)16-5-6-22-23(12-16)35-15-34-22/h1-6,11-12,14H,7-10,13,15H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055887
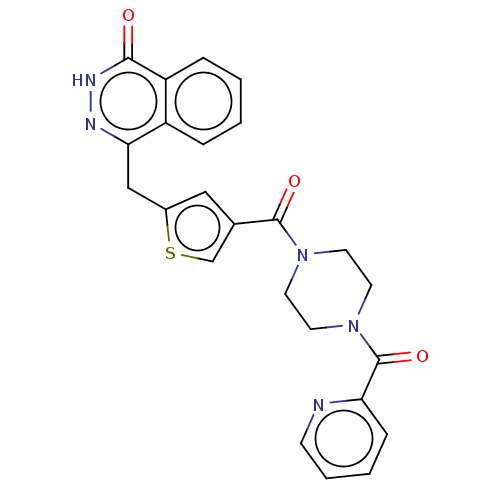
(CHEMBL3317917)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccccn1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C24H21N5O3S/c30-22-19-6-2-1-5-18(19)21(26-27-22)14-17-13-16(15-33-17)23(31)28-9-11-29(12-10-28)24(32)20-7-3-4-8-25-20/h1-8,13,15H,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465347

(CHEBI:52486 | POMIFERIN A)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(C)CO)C(C)C |r| Show InChI InChI=1S/C20H30O/c1-14(2)15-6-8-17-16(12-15)7-9-18-19(3,13-21)10-5-11-20(17,18)4/h6,8,12,14,18,21H,5,7,9-11,13H2,1-4H3/t18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465338

(15-Hydroxydehydroabietic Acid | CHEMBL598566)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(C)C(O)=O)C(C)(C)O |r| Show InChI InChI=1S/C20H28O3/c1-18(2,23)14-7-8-15-13(12-14)6-9-16-19(15,3)10-5-11-20(16,4)17(21)22/h7-8,12,16,23H,5-6,9-11H2,1-4H3,(H,21,22)/t16-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055891

(CHEMBL3317909)Show SMILES Brc1ccc(cc1)C(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C25H21BrN4O3S/c26-18-7-5-16(6-8-18)24(32)29-9-11-30(12-10-29)25(33)17-13-19(34-15-17)14-22-20-3-1-2-4-21(20)23(31)28-27-22/h1-8,13,15H,9-12,14H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50283690
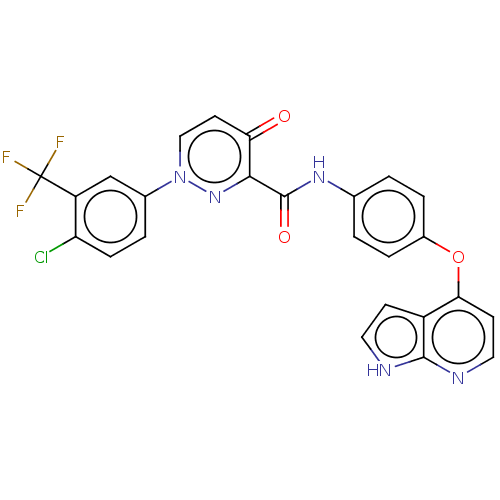
(CHEMBL4165199)Show SMILES FC(F)(F)c1cc(ccc1Cl)-n1ccc(=O)c(n1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)cc1 Show InChI InChI=1S/C25H15ClF3N5O3/c26-19-6-3-15(13-18(19)25(27,28)29)34-12-9-20(35)22(33-34)24(36)32-14-1-4-16(5-2-14)37-21-8-11-31-23-17(21)7-10-30-23/h1-13H,(H,30,31)(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by mobility shift... |
Eur J Med Chem 141: 538-551 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.027
BindingDB Entry DOI: 10.7270/Q2V98BMQ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055884

(CHEMBL3317900)Show SMILES CCC(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C21H22N4O3S/c1-2-19(26)24-7-9-25(10-8-24)21(28)14-11-15(29-13-14)12-18-16-5-3-4-6-17(16)20(27)23-22-18/h3-6,11,13H,2,7-10,12H2,1H3,(H,23,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465343

(CHEMBL4279751)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C20H26O3/c1-13(2)14-5-7-16-15(11-14)6-8-17-19(16,3)9-4-10-20(17,12-21)18(22)23/h5,7,11,17,21H,1,4,6,8-10,12H2,2-3H3,(H,22,23)/t17-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055886
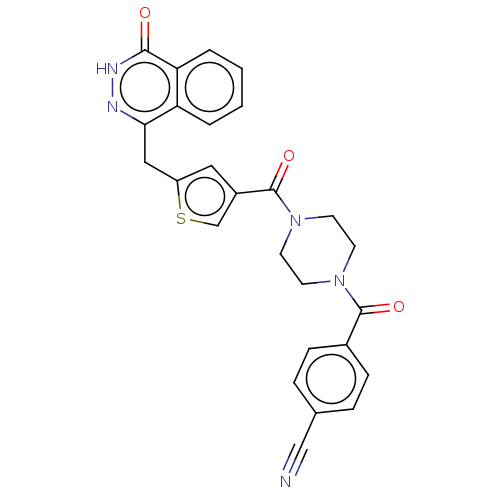
(CHEMBL3317906)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccc(cc1)C#N)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H21N5O3S/c27-15-17-5-7-18(8-6-17)25(33)30-9-11-31(12-10-30)26(34)19-13-20(35-16-19)14-23-21-3-1-2-4-22(21)24(32)29-28-23/h1-8,13,16H,9-12,14H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055885
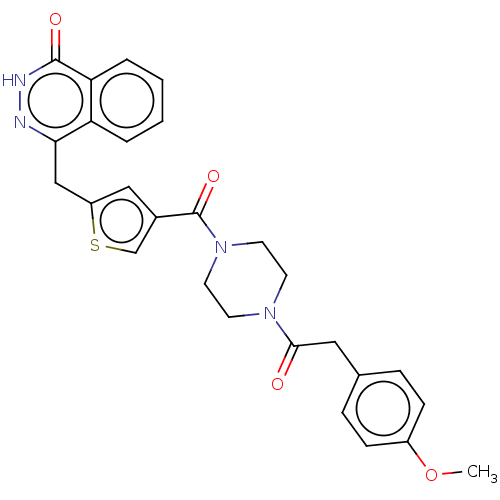
(CHEMBL3317902)Show SMILES COc1ccc(CC(=O)N2CCN(CC2)C(=O)c2csc(Cc3n[nH]c(=O)c4ccccc34)c2)cc1 Show InChI InChI=1S/C27H26N4O4S/c1-35-20-8-6-18(7-9-20)14-25(32)30-10-12-31(13-11-30)27(34)19-15-21(36-17-19)16-24-22-4-2-3-5-23(22)26(33)29-28-24/h2-9,15,17H,10-14,16H2,1H3,(H,29,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465344
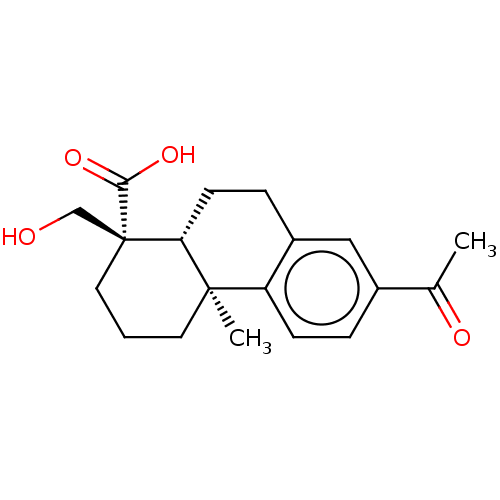
(CHEMBL4294902)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)=O |r| Show InChI InChI=1S/C19H24O4/c1-12(21)13-4-6-15-14(10-13)5-7-16-18(15,2)8-3-9-19(16,11-20)17(22)23/h4,6,10,16,20H,3,5,7-9,11H2,1-2H3,(H,22,23)/t16-,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465343

(CHEMBL4279751)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C20H26O3/c1-13(2)14-5-7-16-15(11-14)6-8-17-19(16,3)9-4-10-20(17,12-21)18(22)23/h5,7,11,17,21H,1,4,6,8-10,12H2,2-3H3,(H,22,23)/t17-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465342

(CHEMBL4293812)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C22H28O4/c1-14(2)15-6-8-17-16(12-15)7-9-18-21(17,3)10-5-11-22(18,20(24)25)13-19(23)26-4/h6,8,12,18H,1,5,7,9-11,13H2,2-4H3,(H,24,25)/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465340

(CHEMBL4278579)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)=O |r| Show InChI InChI=1S/C21H26O5/c1-13(22)14-5-7-16-15(11-14)6-8-17-20(16,2)9-4-10-21(17,19(24)25)12-18(23)26-3/h5,7,11,17H,4,6,8-10,12H2,1-3H3,(H,24,25)/t17-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465339
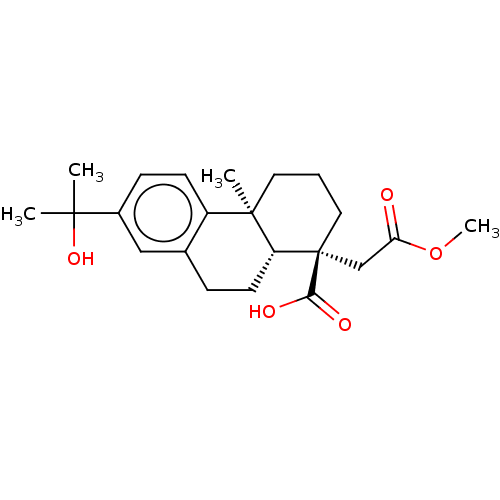
(CHEMBL4285076)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)(C)O |r| Show InChI InChI=1S/C22H30O5/c1-20(2,26)15-7-8-16-14(12-15)6-9-17-21(16,3)10-5-11-22(17,19(24)25)13-18(23)27-4/h7-8,12,17,26H,5-6,9-11,13H2,1-4H3,(H,24,25)/t17-,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465341
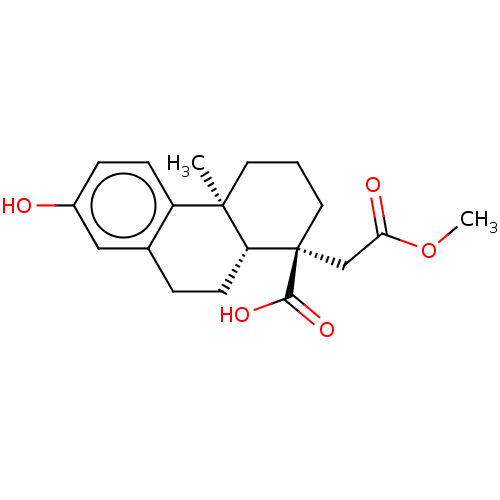
(CHEMBL4289234)Show SMILES [H][C@@]12CCc3cc(O)ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O |r| Show InChI InChI=1S/C19H24O5/c1-18-8-3-9-19(17(22)23,11-16(21)24-2)15(18)7-4-12-10-13(20)5-6-14(12)18/h5-6,10,15,20H,3-4,7-9,11H2,1-2H3,(H,22,23)/t15-,18-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465338

(15-Hydroxydehydroabietic Acid | CHEMBL598566)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(C)C(O)=O)C(C)(C)O |r| Show InChI InChI=1S/C20H28O3/c1-18(2,23)14-7-8-15-13(12-14)6-9-16-19(15,3)10-5-11-20(16,4)17(21)22/h7-8,12,16,23H,5-6,9-11H2,1-4H3,(H,21,22)/t16-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465339

(CHEMBL4285076)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)(C)O |r| Show InChI InChI=1S/C22H30O5/c1-20(2,26)15-7-8-16-14(12-15)6-9-17-21(16,3)10-5-11-22(17,19(24)25)13-18(23)27-4/h7-8,12,17,26H,5-6,9-11,13H2,1-4H3,(H,24,25)/t17-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465348

(CHEMBL4292650)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)C |r| Show InChI InChI=1S/C22H30O4/c1-14(2)15-6-8-17-16(12-15)7-9-18-21(17,3)10-5-11-22(18,20(24)25)13-19(23)26-4/h6,8,12,14,18H,5,7,9-11,13H2,1-4H3,(H,24,25)/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465345

(18-Oxoferruginol | acs.jmedchem.1c00409_ST.768)Show SMILES [H][C@@]12CCc3cc(C(C)C)c(O)cc3[C@@]1(C)CCC[C@@]2(C)C=O |r| Show InChI InChI=1S/C20H28O2/c1-13(2)15-10-14-6-7-18-19(3,12-21)8-5-9-20(18,4)16(14)11-17(15)22/h10-13,18,22H,5-9H2,1-4H3/t18-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50465346

(CHEMBL4286991)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)C |r| Show InChI InChI=1S/C20H28O3/c1-13(2)14-5-7-16-15(11-14)6-8-17-19(16,3)9-4-10-20(17,12-21)18(22)23/h5,7,11,13,17,21H,4,6,8-10,12H2,1-3H3,(H,22,23)/t17-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50143600

(CHEBI:29571 | Dehydroabietic Acid)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(C)C(O)=O)C(C)C |r| Show InChI InChI=1S/C20H28O2/c1-13(2)14-6-8-16-15(12-14)7-9-17-19(16,3)10-5-11-20(17,4)18(21)22/h6,8,12-13,17H,5,7,9-11H2,1-4H3,(H,21,22)/t17-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465344

(CHEMBL4294902)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@]2(CO)C(O)=O)C(C)=O |r| Show InChI InChI=1S/C19H24O4/c1-12(21)13-4-6-15-14(10-13)5-7-16-18(15,2)8-3-9-19(16,11-20)17(22)23/h4,6,10,16,20H,3,5,7-9,11H2,1-2H3,(H,22,23)/t16-,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465341

(CHEMBL4289234)Show SMILES [H][C@@]12CCc3cc(O)ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O |r| Show InChI InChI=1S/C19H24O5/c1-18-8-3-9-19(17(22)23,11-16(21)24-2)15(18)7-4-12-10-13(20)5-6-14(12)18/h5-6,10,15,20H,3-4,7-9,11H2,1-2H3,(H,22,23)/t15-,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50465340

(CHEMBL4278579)Show SMILES [H][C@@]12CCc3cc(ccc3[C@@]1(C)CCC[C@@]2(CC(=O)OC)C(O)=O)C(C)=O |r| Show InChI InChI=1S/C21H26O5/c1-13(22)14-5-7-16-15(11-14)6-8-17-20(16,2)9-4-10-21(17,19(24)25)12-18(23)26-3/h5,7,11,17H,4,6,8-10,12H2,1-3H3,(H,24,25)/t17-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) by mobility shift assay |
J Nat Prod 81: 998-1006 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01082
BindingDB Entry DOI: 10.7270/Q2P84FK3 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055865

(CHEMBL3317897)Show SMILES O=C(C1CC1)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C22H22N4O3S/c27-20-18-4-2-1-3-17(18)19(23-24-20)12-16-11-15(13-30-16)22(29)26-9-7-25(8-10-26)21(28)14-5-6-14/h1-4,11,13-14H,5-10,12H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055888
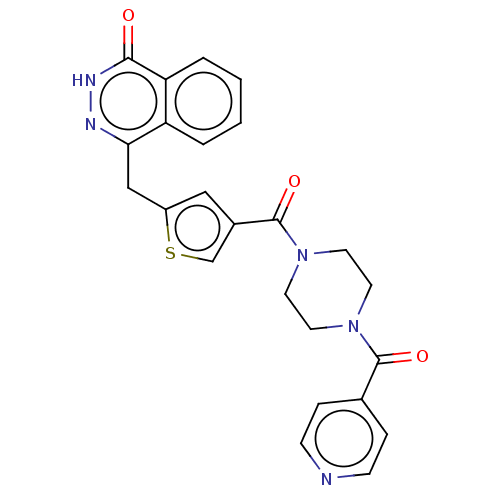
(CHEMBL3317916)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccncc1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C24H21N5O3S/c30-22-20-4-2-1-3-19(20)21(26-27-22)14-18-13-17(15-33-18)24(32)29-11-9-28(10-12-29)23(31)16-5-7-25-8-6-16/h1-8,13,15H,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055883

(CHEMBL3317899)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C20H20N4O3S/c1-13(25)23-6-8-24(9-7-23)20(27)14-10-15(28-12-14)11-18-16-4-2-3-5-17(16)19(26)22-21-18/h2-5,10,12H,6-9,11H2,1H3,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50283693
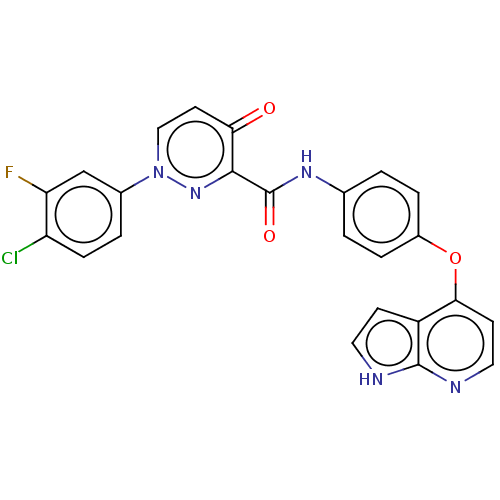
(CHEMBL4169694)Show SMILES Fc1cc(ccc1Cl)-n1ccc(=O)c(n1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)cc1 Show InChI InChI=1S/C24H15ClFN5O3/c25-18-6-3-15(13-19(18)26)31-12-9-20(32)22(30-31)24(33)29-14-1-4-16(5-2-14)34-21-8-11-28-23-17(21)7-10-27-23/h1-13H,(H,27,28)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by mobility shift... |
Eur J Med Chem 141: 538-551 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.027
BindingDB Entry DOI: 10.7270/Q2V98BMQ |
More data for this
Ligand-Target Pair | |
Endo-beta-N-acetylglucosaminidase
(Arthrobacter protophormiae) | BDBM50373151
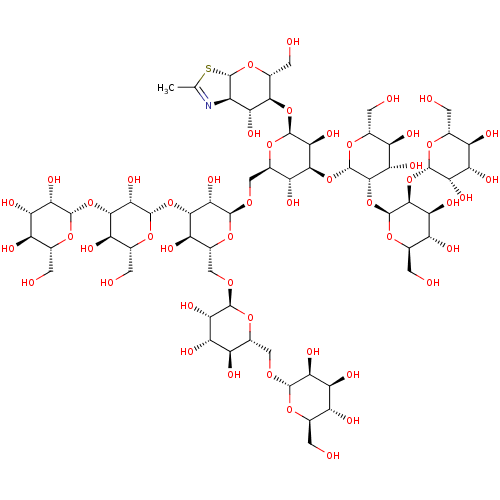
(CHEMBL259756)Show SMILES CC1=N[C@H]2[C@@H](O[C@H](CO)[C@@H](O[C@@H]3O[C@H](CO[C@H]4O[C@H](CO[C@H]5O[C@H](CO[C@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]6O)[C@@H](O)[C@H](O)[C@@H]5O)[C@@H](O)[C@H](O[C@@H]5O[C@H](CO)[C@@H](O)[C@H](O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]6O)[C@@H]5O)[C@@H]4O)[C@@H](O)[C@H](O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]4O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]4O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]4O)[C@@H]3O)[C@@H]2O)S1 |t:1| Show InChI InChI=1S/C62H103NO49S/c1-12-63-23-33(80)47(19(8-70)106-62(23)113-12)107-59-46(93)50(110-60-52(39(86)28(75)16(5-67)101-60)112-61-51(38(85)27(74)17(6-68)102-61)111-57-43(90)36(83)26(73)15(4-66)99-57)32(79)22(105-59)11-96-55-44(91)49(109-58-45(92)48(30(77)18(7-69)100-58)108-56-42(89)35(82)25(72)14(3-65)98-56)31(78)21(104-55)10-95-54-41(88)37(84)29(76)20(103-54)9-94-53-40(87)34(81)24(71)13(2-64)97-53/h13-62,64-93H,2-11H2,1H3/t13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34+,35+,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,62+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of Arthrobacter protophormiae endo-beta-N-acetylglucosaminidase after 5 mins by HPAEC-PED method |
Bioorg Med Chem 16: 4670-5 (2008)
Article DOI: 10.1016/j.bmc.2008.02.032
BindingDB Entry DOI: 10.7270/Q2QJ7J55 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data