 Found 168 hits with Last Name = 'wang' and Initial = 'mm'
Found 168 hits with Last Name = 'wang' and Initial = 'mm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952
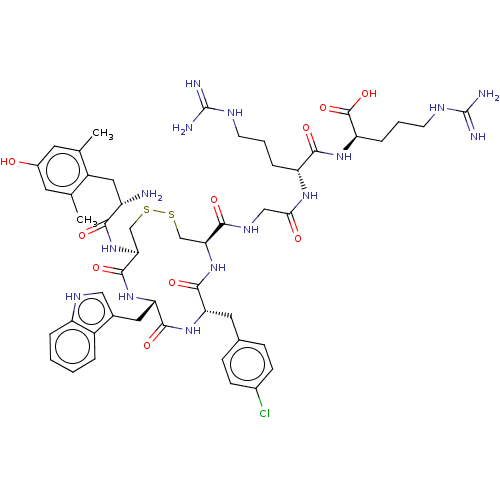
(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953
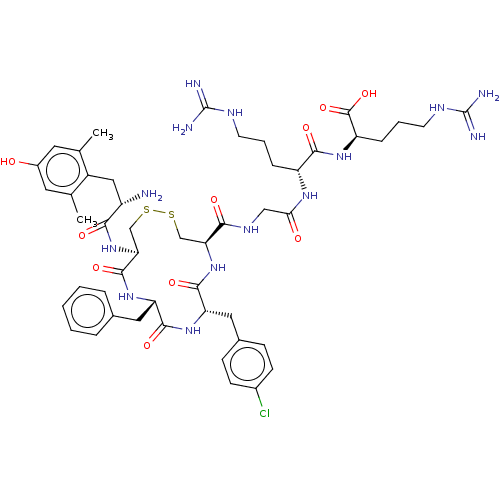
(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951
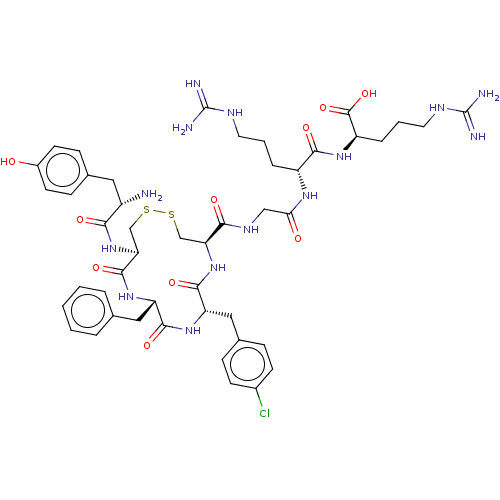
(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952

(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952

(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953

(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953

(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50452815

(CHEMBL2005280)Show InChI InChI=1S/C12H21N3O6/c16-10(17)7-13-1-2-14(8-11(18)19)5-6-15(4-3-13)9-12(20)21/h1-9H2,(H,16,17)(H,18,19)(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of NDM1 in Escherichia coli BL21 (DE3) using MEPM substrate incubated for 15 mins by UV spectroscopy based L-B plot |
Bioorg Med Chem Lett 28: 214-221 (2018)
Article DOI: 10.1016/j.bmcl.2017.10.074
BindingDB Entry DOI: 10.7270/Q20R9S04 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50452814

(CHEMBL4212352)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#16-]-[#6](=S)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](-[#16-])=S)-[#6](-[#16-])=S Show InChI InChI=1S/C9H15N3S6/c13-7(14)10-1-2-11(8(15)16)5-6-12(4-3-10)9(17)18/h1-6H2,(H,13,14)(H,15,16)(H,17,18)/p-3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of NDM1 in Escherichia coli BL21 (DE3) using MEPM substrate incubated for 15 mins by UV spectroscopy based L-B plot |
Bioorg Med Chem Lett 28: 214-221 (2018)
Article DOI: 10.1016/j.bmcl.2017.10.074
BindingDB Entry DOI: 10.7270/Q20R9S04 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951

(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951

(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50452815

(CHEMBL2005280)Show InChI InChI=1S/C12H21N3O6/c16-10(17)7-13-1-2-14(8-11(18)19)5-6-15(4-3-13)9-12(20)21/h1-9H2,(H,16,17)(H,18,19)(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of NDM1 in Escherichia coli BL21 (DE3) using MEPM substrate incubated for 15 mins by UV spectroscopy |
Bioorg Med Chem Lett 28: 214-221 (2018)
Article DOI: 10.1016/j.bmcl.2017.10.074
BindingDB Entry DOI: 10.7270/Q20R9S04 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593885
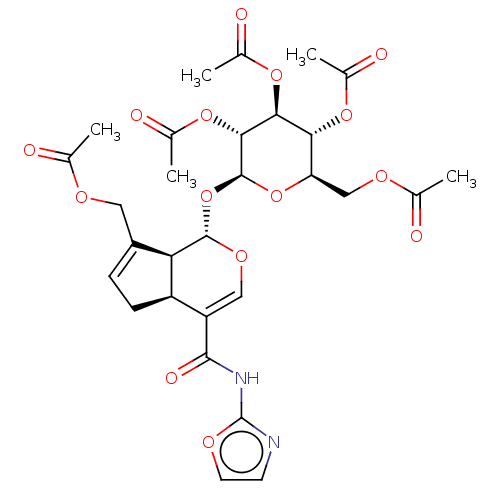
(CHEMBL5195751)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nc1ncco1 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593892

(CHEMBL5182310)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nc1ccc(cc1)C#N |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50441978
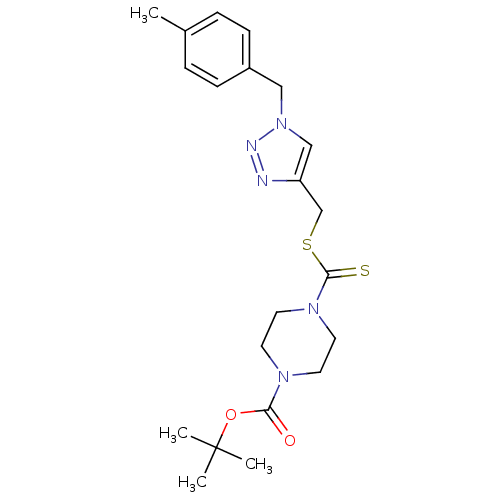
(CHEMBL2334501)Show SMILES Cc1ccc(Cn2cc(CSC(=S)N3CCN(CC3)C(=O)OC(C)(C)C)nn2)cc1 Show InChI InChI=1S/C21H29N5O2S2/c1-16-5-7-17(8-6-16)13-26-14-18(22-23-26)15-30-20(29)25-11-9-24(10-12-25)19(27)28-21(2,3)4/h5-8,14H,9-13,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593883

(CHEMBL5193622)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nn1cccc1 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593889

(CHEMBL5199315)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nc1ccc(Br)c2ccccc12 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593879
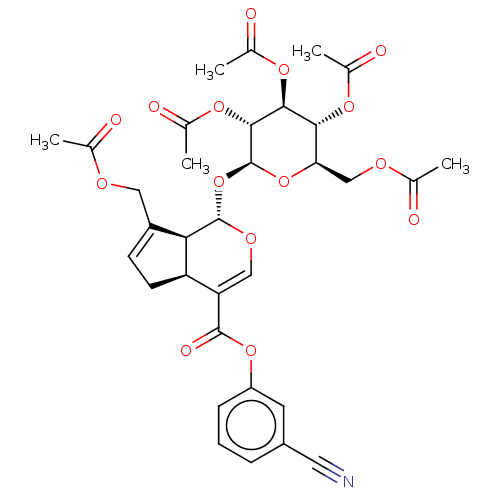
(CHEMBL5188486)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Oc1cccc(c1)C#N |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50452814

(CHEMBL4212352)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#16-]-[#6](=S)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](-[#16-])=S)-[#6](-[#16-])=S Show InChI InChI=1S/C9H15N3S6/c13-7(14)10-1-2-11(8(15)16)5-6-12(4-3-10)9(17)18/h1-6H2,(H,13,14)(H,15,16)(H,17,18)/p-3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of NDM1 in Escherichia coli BL21 (DE3) using MEPM substrate incubated for 15 mins by UV spectroscopy |
Bioorg Med Chem Lett 28: 214-221 (2018)
Article DOI: 10.1016/j.bmcl.2017.10.074
BindingDB Entry DOI: 10.7270/Q20R9S04 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50441979

(CHEMBL2334497)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=S)SCc1cn(Cc2ccccc2F)nn1 Show InChI InChI=1S/C20H26FN5O2S2/c1-20(2,3)28-18(27)24-8-10-25(11-9-24)19(29)30-14-16-13-26(23-22-16)12-15-6-4-5-7-17(15)21/h4-7,13H,8-12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593882
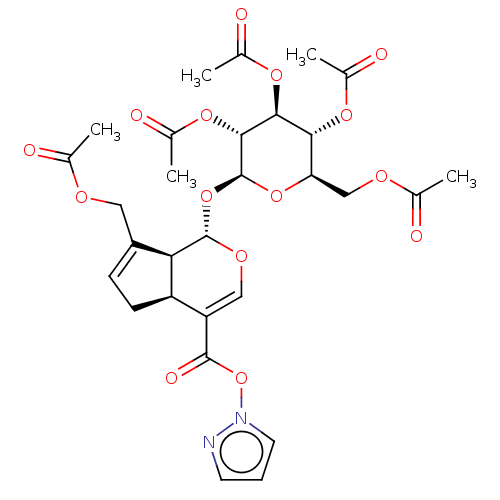
(CHEMBL5199584)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)On1cccn1 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593880
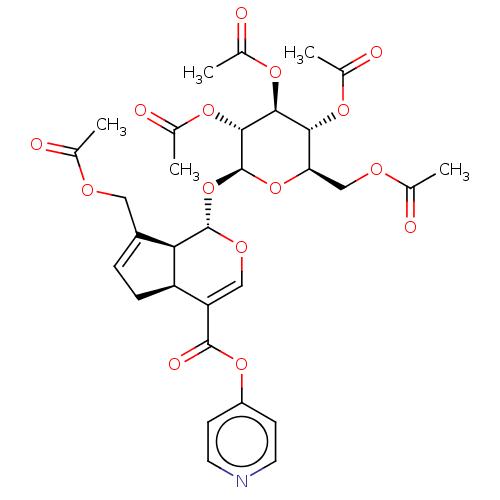
(CHEMBL5188118)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Oc1ccncc1 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442050
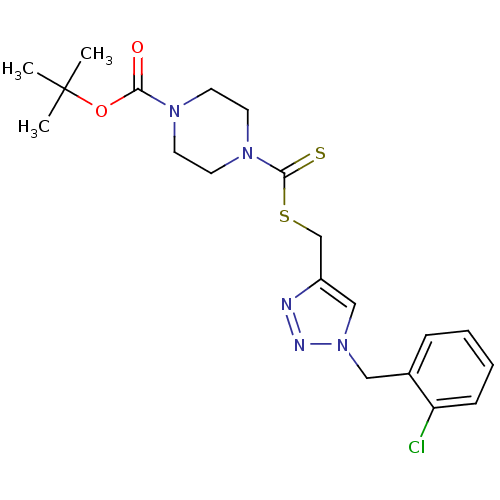
(CHEMBL2334499)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=S)SCc1cn(Cc2ccccc2Cl)nn1 Show InChI InChI=1S/C20H26ClN5O2S2/c1-20(2,3)28-18(27)24-8-10-25(11-9-24)19(29)30-14-16-13-26(23-22-16)12-15-6-4-5-7-17(15)21/h4-7,13H,8-12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50140241

(Allopurinol | Aloral | Aluline 100 | Aluline 300 |...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593877

(CHEMBL5171292)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)SC1=NCCS1 |r,c:41,t:3,47| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593873

(CHEMBL5192231)Show SMILES [H][C@]12CC=C(COC(=O)Nc3ccc(OC)cc3)[C@@]1([H])C(OC(=O)Nc1ccc(OC)cc1)OC=C2C(=O)OC |r,c:37,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442030

(CHEMBL2440574)Show SMILES CC(C)OC(=O)N1CCN(CC1)C(=S)SCc1cn(Cc2ccc(C)cc2)nn1 Show InChI InChI=1S/C20H27N5O2S2/c1-15(2)27-19(26)23-8-10-24(11-9-23)20(28)29-14-18-13-25(22-21-18)12-17-6-4-16(3)5-7-17/h4-7,13,15H,8-12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593888
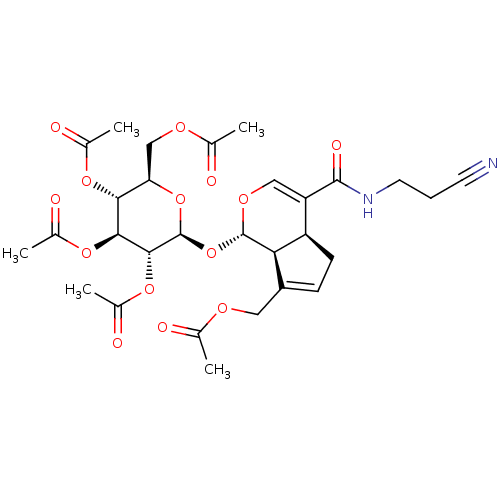
(CHEMBL5172785)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)NCCC#N |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442031
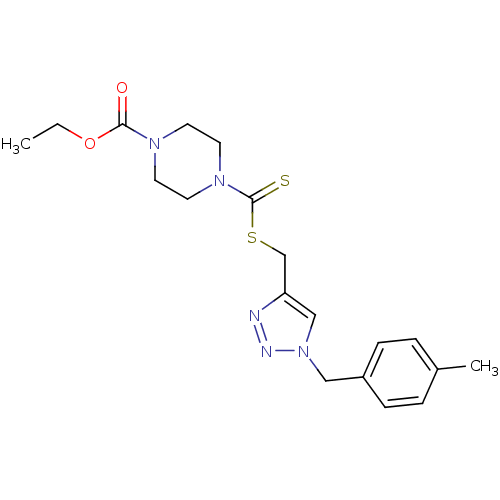
(CHEMBL2440573)Show SMILES CCOC(=O)N1CCN(CC1)C(=S)SCc1cn(Cc2ccc(C)cc2)nn1 Show InChI InChI=1S/C19H25N5O2S2/c1-3-26-18(25)22-8-10-23(11-9-22)19(27)28-14-17-13-24(21-20-17)12-16-6-4-15(2)5-7-16/h4-7,13H,3,8-12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50441980
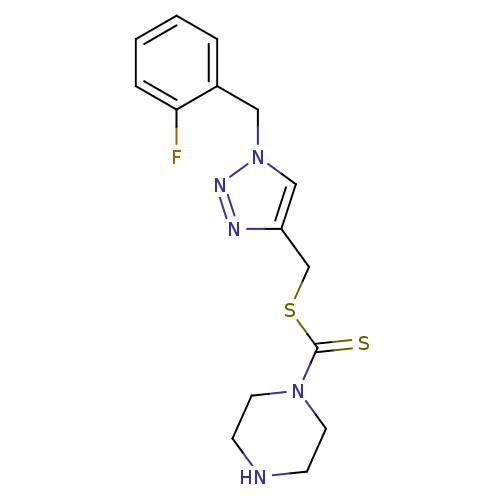
(CHEMBL2334505)Show InChI InChI=1S/C15H18FN5S2/c16-14-4-2-1-3-12(14)9-21-10-13(18-19-21)11-23-15(22)20-7-5-17-6-8-20/h1-4,10,17H,5-9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593887

(CHEMBL5184530)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nc1cccc2[nH]ccc12 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442026
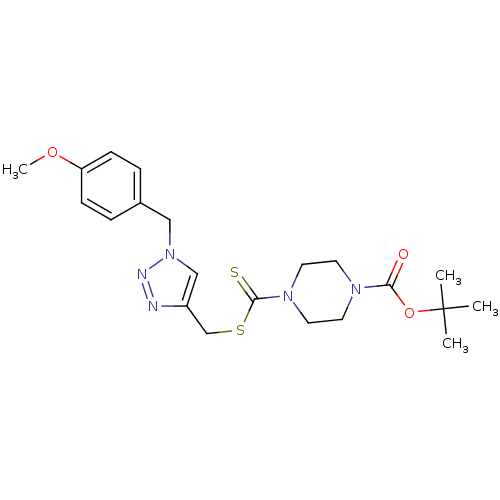
(CHEMBL2334502)Show SMILES COc1ccc(Cn2cc(CSC(=S)N3CCN(CC3)C(=O)OC(C)(C)C)nn2)cc1 Show InChI InChI=1S/C21H29N5O3S2/c1-21(2,3)29-19(27)24-9-11-25(12-10-24)20(30)31-15-17-14-26(23-22-17)13-16-5-7-18(28-4)8-6-16/h5-8,14H,9-13,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50593895

(CHEMBL5203062)Show SMILES [H][C@]12CC=C(COC(C)=O)[C@@]1([H])[C@]([H])(O[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O)OC=C2C(=O)Nc1cncc2ccccc12 |r,c:41,t:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114379
BindingDB Entry DOI: 10.7270/Q21V5JZM |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442013
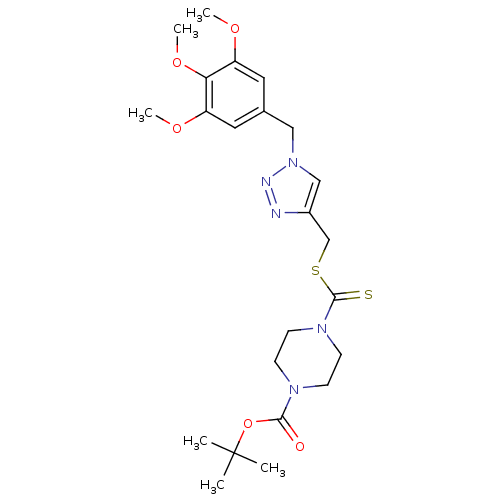
(CHEMBL2334504)Show SMILES COc1cc(Cn2cc(CSC(=S)N3CCN(CC3)C(=O)OC(C)(C)C)nn2)cc(OC)c1OC Show InChI InChI=1S/C23H33N5O5S2/c1-23(2,3)33-21(29)26-7-9-27(10-8-26)22(34)35-15-17-14-28(25-24-17)13-16-11-18(30-4)20(32-6)19(12-16)31-5/h11-12,14H,7-10,13,15H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50442044
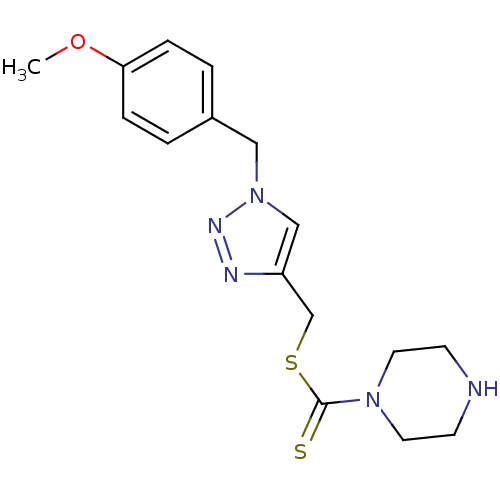
(CHEMBL2334191)Show InChI InChI=1S/C16H21N5OS2/c1-22-15-4-2-13(3-5-15)10-21-11-14(18-19-21)12-24-16(23)20-8-6-17-7-9-20/h2-5,11,17H,6-10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 (157 to 852 aa) expressed in Escherichia coli BL21(DE) using H3K4me2 as substrate by fluorescence assay |
J Med Chem 56: 8543-60 (2013)
Article DOI: 10.1021/jm401002r
BindingDB Entry DOI: 10.7270/Q2XD1333 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data