 Found 29 hits with Last Name = 'weissleder' and Initial = 'r'
Found 29 hits with Last Name = 'weissleder' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50296231
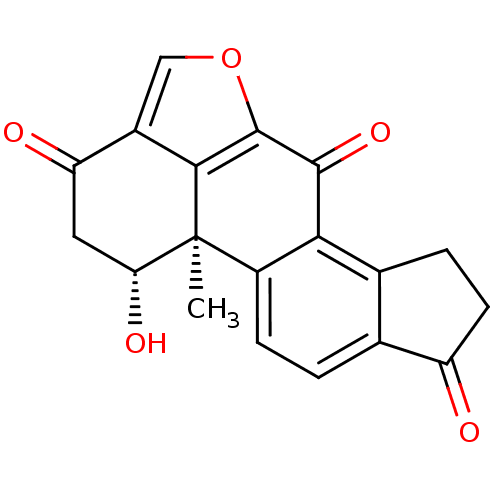
(CHEMBL549959 | demethoxyviridin)Show SMILES C[C@]12[C@H](O)CC(=O)c3coc(c13)C(=O)c1c3CCC(=O)c3ccc21 |r| Show InChI InChI=1S/C19H14O5/c1-19-11-4-2-8-9(3-5-12(8)20)15(11)17(23)18-16(19)10(7-24-18)13(21)6-14(19)22/h2,4,7,14,22H,3,5-6H2,1H3/t14-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50358130
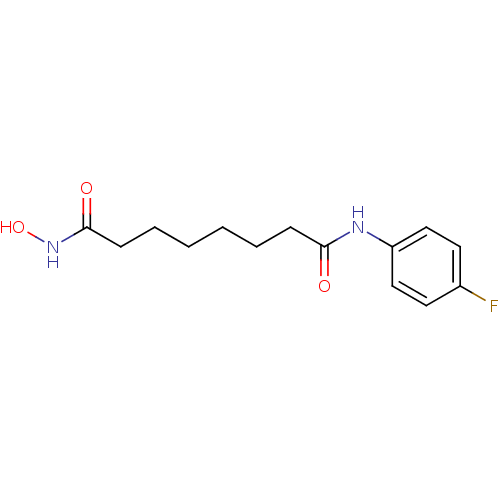
(CHEMBL1923808)Show InChI InChI=1S/C14H19FN2O3/c15-11-7-9-12(10-8-11)16-13(18)5-3-1-2-4-6-14(19)17-20/h7-10,20H,1-6H2,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC1 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC1 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM15234
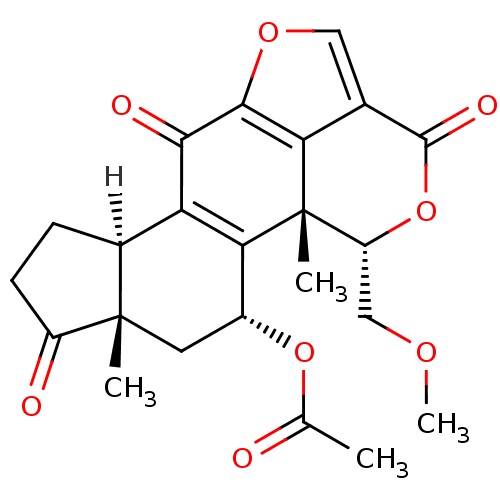
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
| Assay Description
Inhibition of PI3 kinase by WmC20 derivatives causing a formation of covalent bond between WmC20 derivatives and the target protein. |
Chem Biol 14: 321-8 (2007)
Article DOI: 10.1016/j.chembiol.2007.02.007
BindingDB Entry DOI: 10.7270/Q21J986V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM15234

((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50358130

(CHEMBL1923808)Show InChI InChI=1S/C14H19FN2O3/c15-11-7-9-12(10-8-11)16-13(18)5-3-1-2-4-6-14(19)17-20/h7-10,20H,1-6H2,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC2 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC2 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM15234

((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50296232
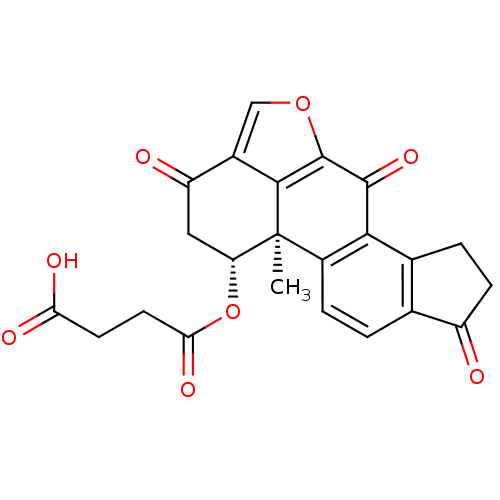
(CHEMBL563654 | Succinic acid mono-((5bR,6R)-5b-met...)Show SMILES C[C@]12[C@@H](CC(=O)c3coc(c13)C(=O)c1c3CCC(=O)c3ccc21)OC(=O)CCC(O)=O |r| Show InChI InChI=1S/C23H18O8/c1-23-13-4-2-10-11(3-5-14(10)24)19(13)21(29)22-20(23)12(9-30-22)15(25)8-16(23)31-18(28)7-6-17(26)27/h2,4,9,16H,3,5-8H2,1H3,(H,26,27)/t16-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC3 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50358130

(CHEMBL1923808)Show InChI InChI=1S/C14H19FN2O3/c15-11-7-9-12(10-8-11)16-13(18)5-3-1-2-4-6-14(19)17-20/h7-10,20H,1-6H2,(H,16,18)(H,17,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC3 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262092
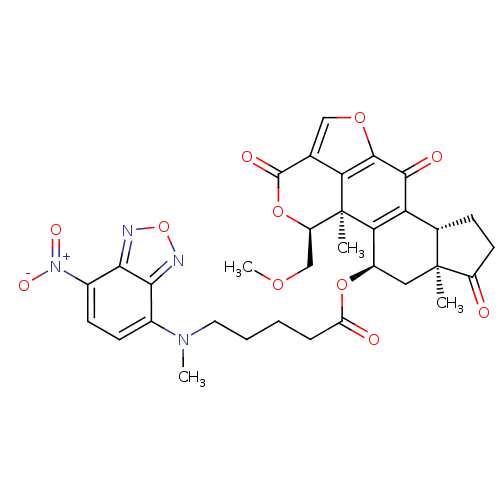
(5-[Methyl-(7-nitro-benzo[1,2,5]oxadiazol-4-yl)-ami...)Show SMILES COC[C@H]1OC(=O)c2coc3c2[C@@]1(C)C1=C([C@@H]2CCC(=O)[C@@]2(C)C[C@H]1OC(=O)CCCCN(C)c1ccc([N+]([O-])=O)c2nonc12)C3=O |r,t:16| Show InChI InChI=1S/C33H34N4O11/c1-32-13-20(46-23(39)7-5-6-12-36(3)18-9-10-19(37(42)43)28-27(18)34-48-35-28)26-24(17(32)8-11-21(32)38)29(40)30-25-16(14-45-30)31(41)47-22(15-44-4)33(25,26)2/h9-10,14,17,20,22H,5-8,11-13,15H2,1-4H3/t17-,20+,22+,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50296233
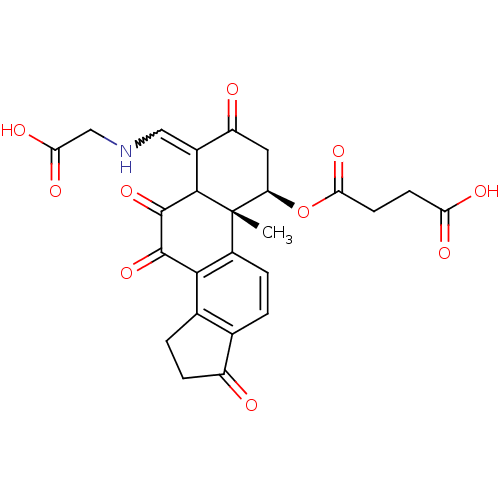
(4-((1R,10R,Z)-4-((carboxymethylamino)methylene)-6-...)Show SMILES C[C@]12[C@@H](CC(=O)C(=CNCC(O)=O)C1C(=O)C(=O)c1c3CCC(=O)c3ccc21)OC(=O)CCC(O)=O |r,w:7.7| Show InChI InChI=1S/C25H23NO10/c1-25-14-4-2-11-12(3-5-15(11)27)21(14)23(34)24(35)22(25)13(9-26-10-19(31)32)16(28)8-17(25)36-20(33)7-6-18(29)30/h2,4,9,17,22,26H,3,5-8,10H2,1H3,(H,29,30)(H,31,32)/t17-,22?,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50358130

(CHEMBL1923808)Show InChI InChI=1S/C14H19FN2O3/c15-11-7-9-12(10-8-11)16-13(18)5-3-1-2-4-6-14(19)17-20/h7-10,20H,1-6H2,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC6 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 57.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC6 using fluorophore conjugated substrate by fluorescence assay |
J Med Chem 54: 5576-82 (2011)
Article DOI: 10.1021/jm200620f
BindingDB Entry DOI: 10.7270/Q2JW8F90 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262097
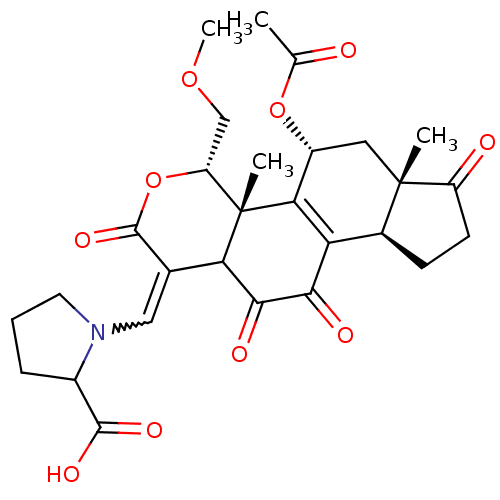
(1-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...)Show SMILES COC[C@H]1OC(=O)C(=CN2CCCC2C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:23| Show InChI InChI=1S/C28H33NO10/c1-13(30)38-17-10-27(2)15(7-8-18(27)31)20-22(17)28(3)19(12-37-4)39-26(36)14(21(28)24(33)23(20)32)11-29-9-5-6-16(29)25(34)35/h11,15-17,19,21H,5-10,12H2,1-4H3,(H,34,35)/t15-,16?,17+,19+,21?,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262097

(1-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...)Show SMILES COC[C@H]1OC(=O)C(=CN2CCCC2C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:23| Show InChI InChI=1S/C28H33NO10/c1-13(30)38-17-10-27(2)15(7-8-18(27)31)20-22(17)28(3)19(12-37-4)39-26(36)14(21(28)24(33)23(20)32)11-29-9-5-6-16(29)25(34)35/h11,15-17,19,21H,5-10,12H2,1-4H3,(H,34,35)/t15-,16?,17+,19+,21?,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
| Assay Description
Inhibition of PI3 kinase by WmC20 derivatives causing a formation of covalent bond between WmC20 derivatives and the target protein. |
Chem Biol 14: 321-8 (2007)
Article DOI: 10.1016/j.chembiol.2007.02.007
BindingDB Entry DOI: 10.7270/Q21J986V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262097

(1-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...)Show SMILES COC[C@H]1OC(=O)C(=CN2CCCC2C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:23| Show InChI InChI=1S/C28H33NO10/c1-13(30)38-17-10-27(2)15(7-8-18(27)31)20-22(17)28(3)19(12-37-4)39-26(36)14(21(28)24(33)23(20)32)11-29-9-5-6-16(29)25(34)35/h11,15-17,19,21H,5-10,12H2,1-4H3,(H,34,35)/t15-,16?,17+,19+,21?,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262095
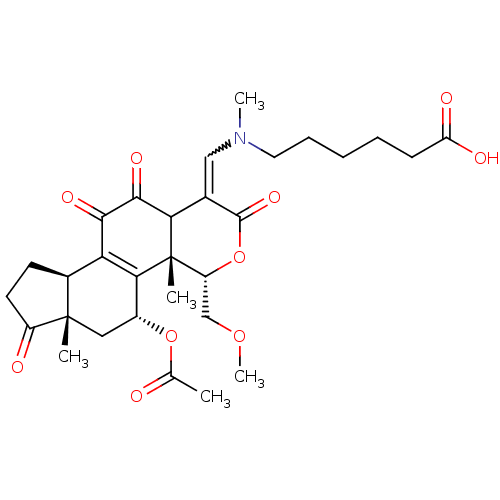
((Z)-6-(((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a...)Show SMILES COC[C@H]1OC(=O)C(=CN(C)CCCCCC(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:24| Show InChI InChI=1S/C30H39NO10/c1-16(32)40-19-13-29(2)18(10-11-20(29)33)23-25(19)30(3)21(15-39-5)41-28(38)17(24(30)27(37)26(23)36)14-31(4)12-8-6-7-9-22(34)35/h14,18-19,21,24H,6-13,15H2,1-5H3,(H,34,35)/t18-,19+,21+,24?,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
| Assay Description
Inhibition of PI3 kinase by WmC20 derivatives causing a formation of covalent bond between WmC20 derivatives and the target protein. |
Chem Biol 14: 321-8 (2007)
Article DOI: 10.1016/j.chembiol.2007.02.007
BindingDB Entry DOI: 10.7270/Q21J986V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262093
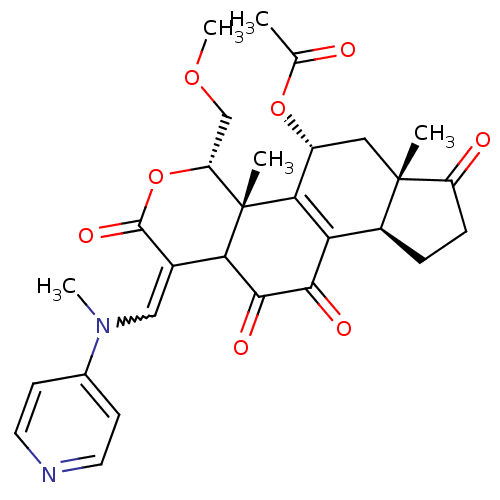
(11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...)Show SMILES COC[C@H]1OC(=O)C(=CN(C)c2ccncc2)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:23| Show InChI InChI=1S/C29H32N2O8/c1-15(32)38-19-12-28(2)18(6-7-20(28)33)22-24(19)29(3)21(14-37-5)39-27(36)17(23(29)26(35)25(22)34)13-31(4)16-8-10-30-11-9-16/h8-11,13,18-19,21,23H,6-7,12,14H2,1-5H3/t18-,19+,21+,23?,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262095

((Z)-6-(((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a...)Show SMILES COC[C@H]1OC(=O)C(=CN(C)CCCCCC(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:24| Show InChI InChI=1S/C30H39NO10/c1-16(32)40-19-13-29(2)18(10-11-20(29)33)23-25(19)30(3)21(15-39-5)41-28(38)17(24(30)27(37)26(23)36)14-31(4)12-8-6-7-9-22(34)35/h14,18-19,21,24H,6-13,15H2,1-5H3,(H,34,35)/t18-,19+,21+,24?,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262096
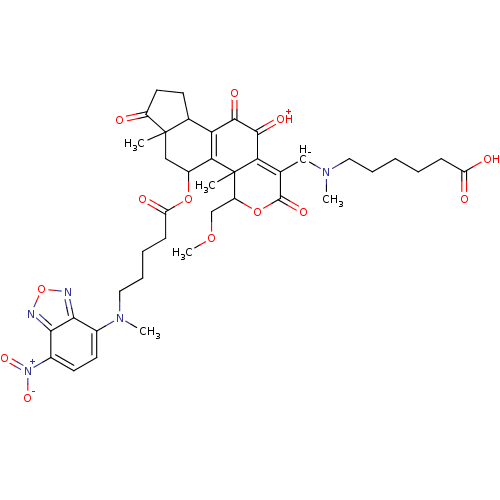
(6-(((11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-5...)Show SMILES COCC1OC(=O)C([CH-]N(C)CCCCCC(O)=O)=C2C(=[OH+])C(=O)C3=C(C(CC4(C)C3CCC4=O)OC(=O)CCCCN(C)c3ccc([N+]([O-])=O)c4nonc34)C12C |t:18,24| Show InChI InChI=1S/C40H48N5O13/c1-39-19-26(56-30(49)12-8-10-18-44(4)24-14-15-25(45(53)54)35-34(24)41-58-42-35)33-31(23(39)13-16-27(39)46)36(50)37(51)32-22(20-43(3)17-9-6-7-11-29(47)48)38(52)57-28(21-55-5)40(32,33)2/h14-15,20,23,26,28H,6-13,16-19,21H2,1-5H3,(H,47,48)/q-1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262094
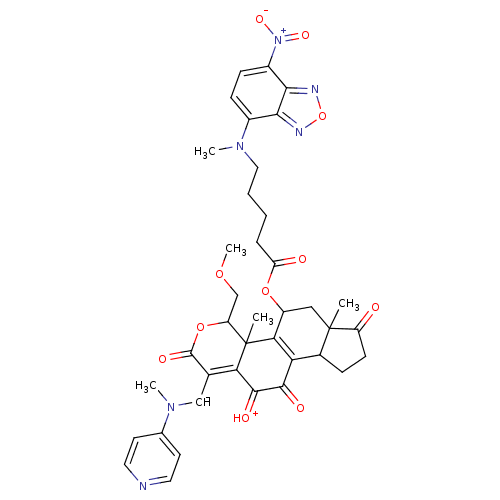
(11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...)Show SMILES COCC1OC(=O)C([CH-]N(C)c2ccncc2)=C2C(=[OH+])C(=O)C3=C(C(CC4(C)C3CCC4=O)OC(=O)CCCCN(C)c3ccc([N+]([O-])=O)c4nonc34)C12C |t:17,23| Show InChI InChI=1S/C39H41N6O11/c1-38-18-26(54-29(47)8-6-7-17-43(3)24-10-11-25(45(51)52)34-33(24)41-56-42-34)32-30(23(38)9-12-27(38)46)35(48)36(49)31-22(19-44(4)21-13-15-40-16-14-21)37(50)55-28(20-53-5)39(31,32)2/h10-11,13-16,19,23,26,28H,6-9,12,17-18,20H2,1-5H3/q-1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262139
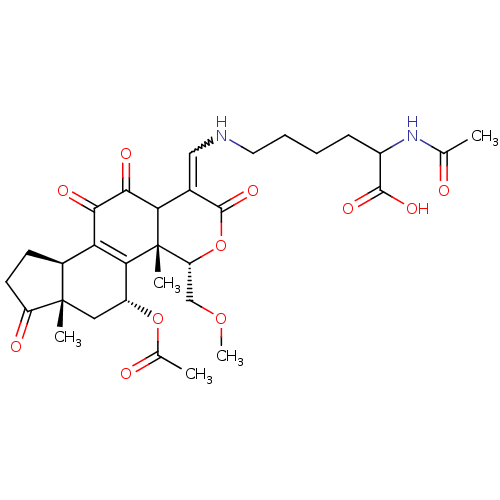
(2-acetamido-6-((5-acetoxy-11-hydroxy-4-(methoxymet...)Show SMILES COC[C@H]1OC(=O)C(=CNCCCCC(NC(C)=O)C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:27| Show InChI InChI=1S/C31H40N2O11/c1-15(34)33-19(28(39)40)8-6-7-11-32-13-17-24-27(38)26(37)23-18-9-10-21(36)30(18,3)12-20(43-16(2)35)25(23)31(24,4)22(14-42-5)44-29(17)41/h13,18-20,22,24,32H,6-12,14H2,1-5H3,(H,33,34)(H,39,40)/t18-,19?,20+,22+,24?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3 kinase |
Bioorg Med Chem Lett 19: 4223-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.105
BindingDB Entry DOI: 10.7270/Q2QZ2B0P |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262139

(2-acetamido-6-((5-acetoxy-11-hydroxy-4-(methoxymet...)Show SMILES COC[C@H]1OC(=O)C(=CNCCCCC(NC(C)=O)C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:27| Show InChI InChI=1S/C31H40N2O11/c1-15(34)33-19(28(39)40)8-6-7-11-32-13-17-24-27(38)26(37)23-18-9-10-21(36)30(18,3)12-20(43-16(2)35)25(23)31(24,4)22(14-42-5)44-29(17)41/h13,18-20,22,24,32H,6-12,14H2,1-5H3,(H,33,34)(H,39,40)/t18-,19?,20+,22+,24?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
| Assay Description
Inhibition of PI3 kinase by WmC20 derivatives causing a formation of covalent bond between WmC20 derivatives and the target protein. |
Chem Biol 14: 321-8 (2007)
Article DOI: 10.1016/j.chembiol.2007.02.007
BindingDB Entry DOI: 10.7270/Q21J986V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM68273
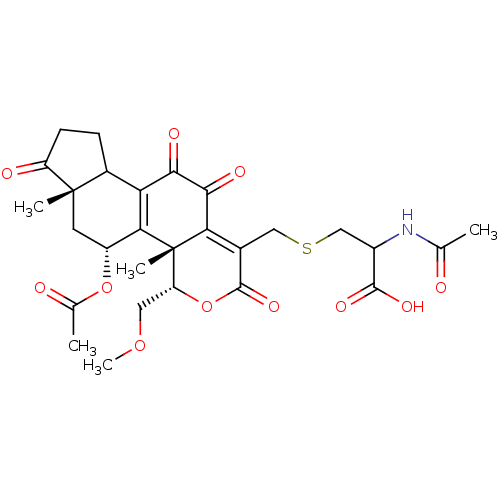
(WmC20-cys, 3)Show SMILES COC[C@H]1OC(=O)C(CSCC(NC(C)=O)C(O)=O)=C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)C3CCC4=O)OC(C)=O)[C@@]12C |r,t:18,24| Show InChI InChI=1S/C28H33NO11S/c1-12(30)29-16(25(35)36)11-41-10-14-21-24(34)23(33)20-15-6-7-18(32)27(15,3)8-17(39-13(2)31)22(20)28(21,4)19(9-38-5)40-26(14)37/h15-17,19H,6-11H2,1-5H3,(H,29,30)(H,35,36)/t15?,16?,17-,19-,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
| Assay Description
Inhibition of PI3 kinase by WmC20 derivatives causing a formation of covalent bond between WmC20 derivatives and the target protein. |
Chem Biol 14: 321-8 (2007)
Article DOI: 10.1016/j.chembiol.2007.02.007
BindingDB Entry DOI: 10.7270/Q21J986V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262138
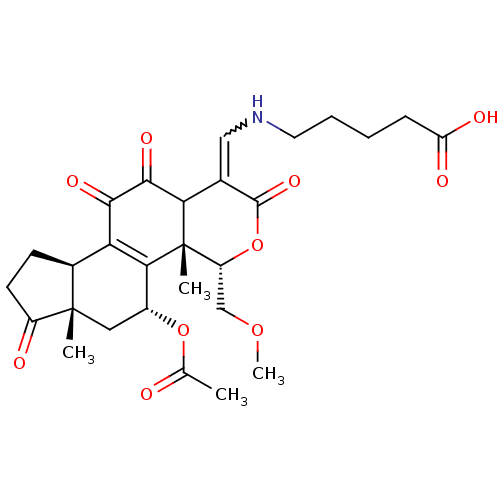
(5-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...)Show SMILES COC[C@H]1OC(=O)C(=CNCCCCC(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:22| Show InChI InChI=1S/C28H35NO10/c1-14(30)38-17-11-27(2)16(8-9-18(27)31)21-23(17)28(3)19(13-37-4)39-26(36)15(22(28)25(35)24(21)34)12-29-10-6-5-7-20(32)33/h12,16-17,19,22,29H,5-11,13H2,1-4H3,(H,32,33)/t16-,17+,19+,22?,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262139

(2-acetamido-6-((5-acetoxy-11-hydroxy-4-(methoxymet...)Show SMILES COC[C@H]1OC(=O)C(=CNCCCCC(NC(C)=O)C(O)=O)C2C(=O)C(=O)C3=C([C@@H](C[C@@]4(C)[C@H]3CCC4=O)OC(C)=O)[C@@]12C |r,w:8.8,t:27| Show InChI InChI=1S/C31H40N2O11/c1-15(34)33-19(28(39)40)8-6-7-11-32-13-17-24-27(38)26(37)23-18-9-10-21(36)30(18,3)12-20(43-16(2)35)25(23)31(24,4)22(14-42-5)44-29(17)41/h13,18-20,22,24,32H,6-12,14H2,1-5H3,(H,33,34)(H,39,40)/t18-,19?,20+,22+,24?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50262043
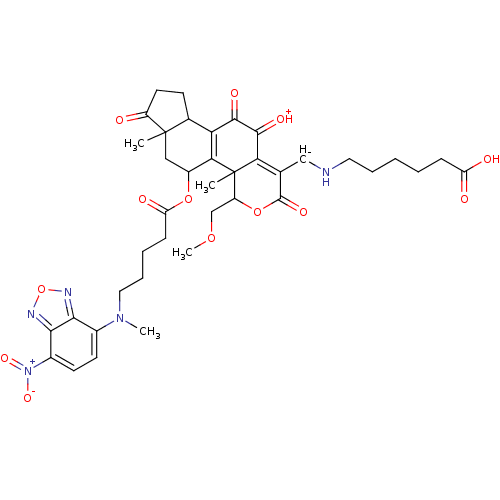
(6-((11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-5-...)Show SMILES COCC1OC(=O)C([CH-]NCCCCCC(O)=O)=C2C(=[OH+])C(=O)C3=C(C(CC4(C)C3CCC4=O)OC(=O)CCCCN(C)c3ccc([N+]([O-])=O)c4nonc34)C12C |t:17,23| Show InChI InChI=1S/C39H46N5O13/c1-38-18-25(55-29(48)11-7-9-17-43(3)23-13-14-24(44(52)53)34-33(23)41-57-42-34)32-30(22(38)12-15-26(38)45)35(49)36(50)31-21(19-40-16-8-5-6-10-28(46)47)37(51)56-27(20-54-4)39(31,32)2/h13-14,19,22,25,27,40H,5-12,15-18,20H2,1-4H3,(H,46,47)/q-1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay |
J Med Chem 51: 4699-707 (2008)
Article DOI: 10.1021/jm800374f
BindingDB Entry DOI: 10.7270/Q22B8XV3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data