

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423171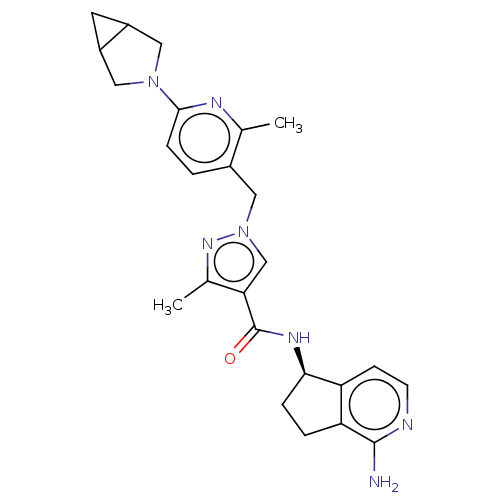 (US10501440, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423169 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423170 (US10501440, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423183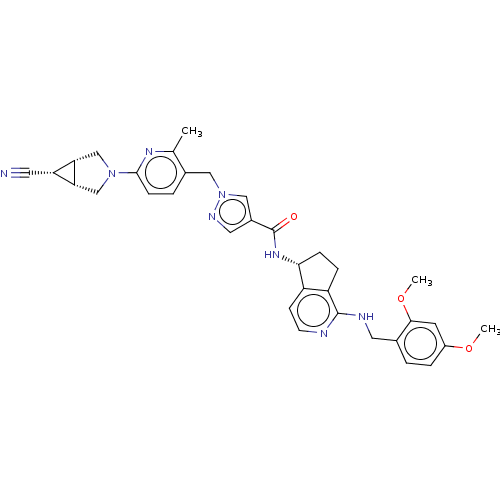 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423185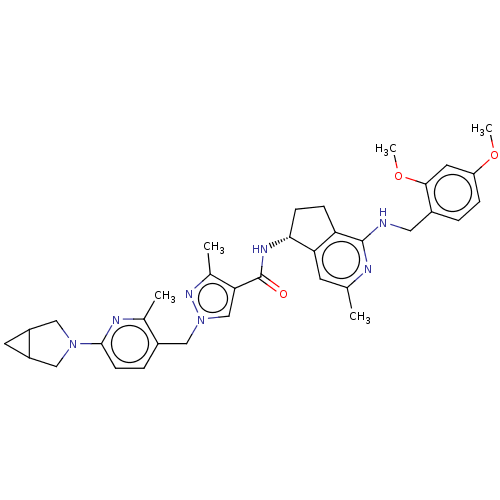 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423174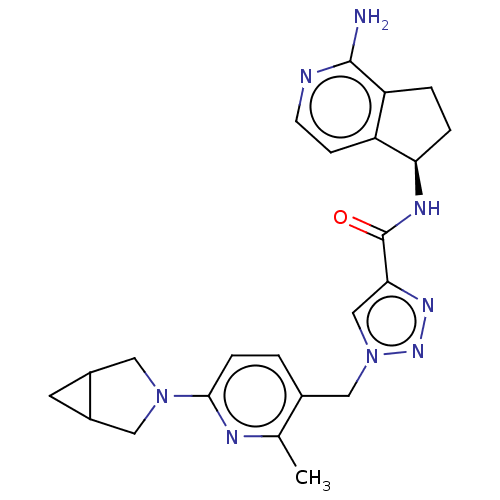 (US10501440, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423179 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423180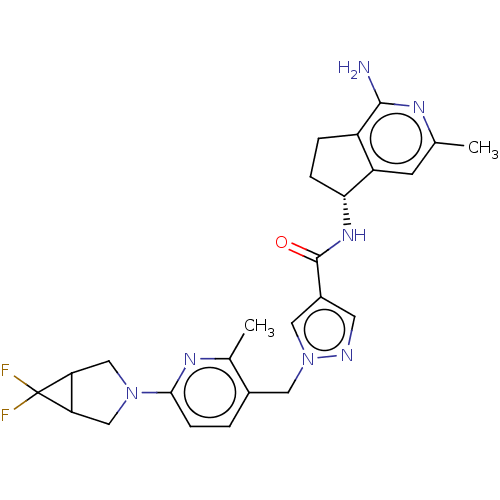 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423173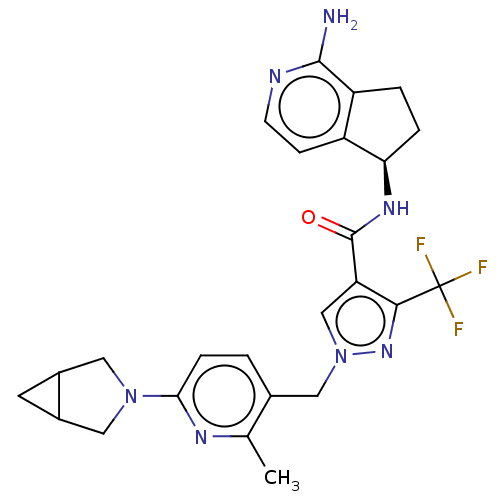 (US10501440, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423188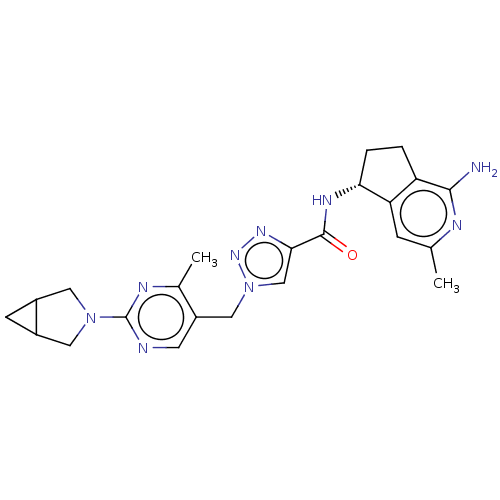 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423187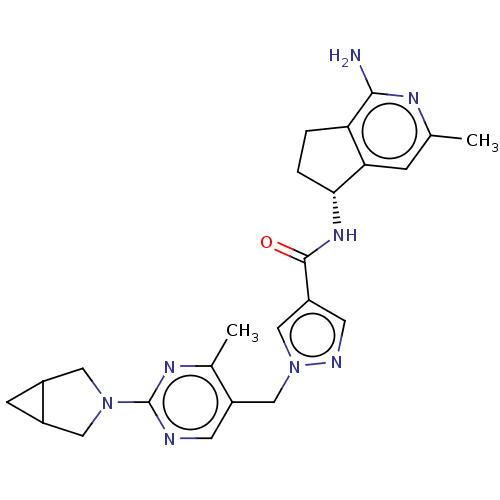 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423182 (US10501440, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423184 (US10501440, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423181 (US10501440, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423176 (US10501440, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM423186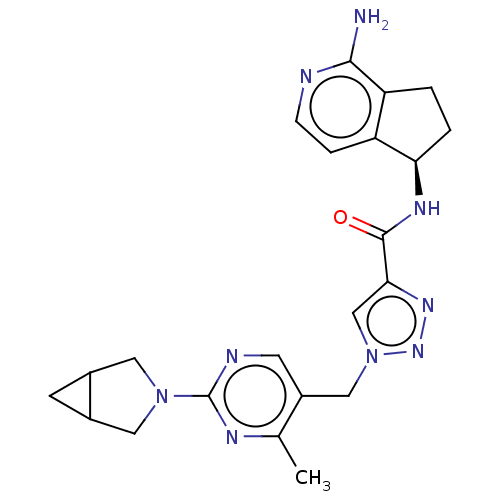 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135146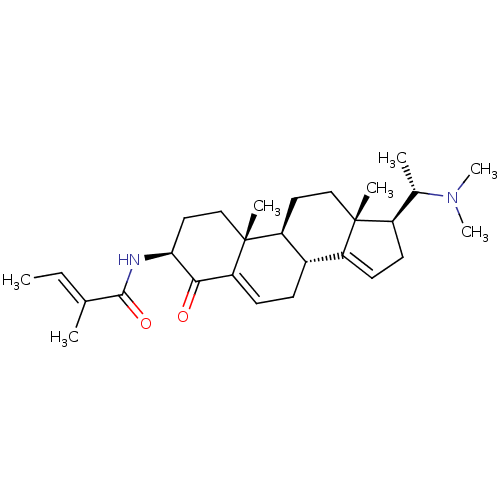 ((E)-2-Methyl-but-2-enoic acid [(3S,8R,9S,10R,13R,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135147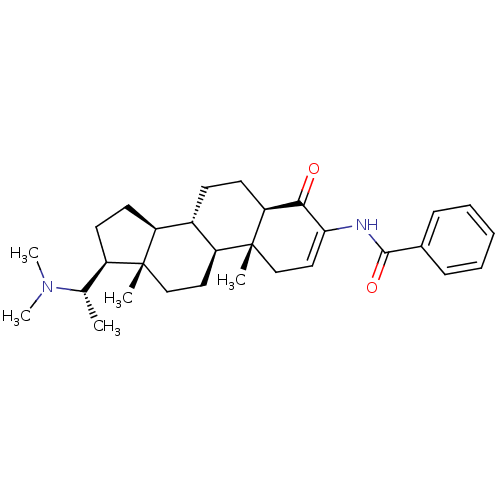 ((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135157 (Acetic acid (1S,6R,7R,10R,11S,12S,15S,16S)-17-((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135146 ((E)-2-Methyl-but-2-enoic acid [(3S,8R,9S,10R,13R,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135147 ((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135158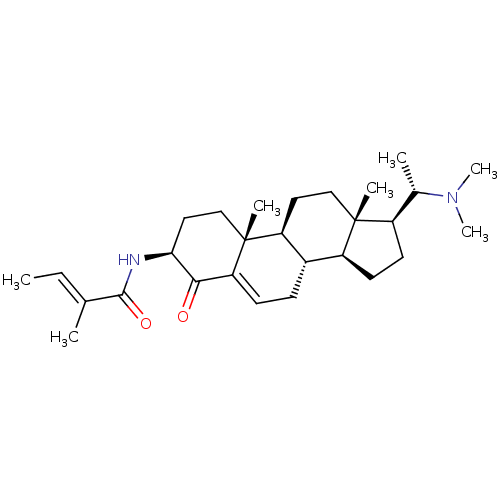 ((E)-2-Methyl-but-2-enoic acid [(3S,8S,9S,10R,13S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135155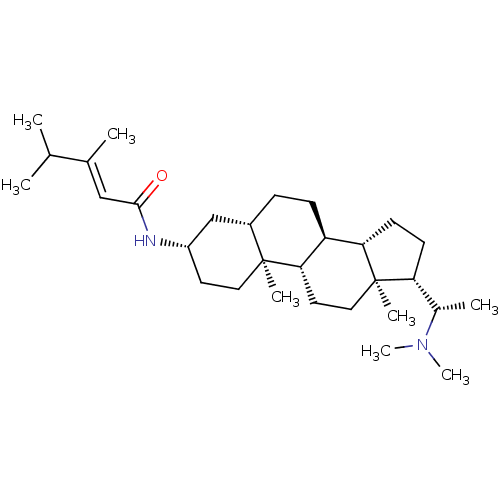 (3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,10S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135153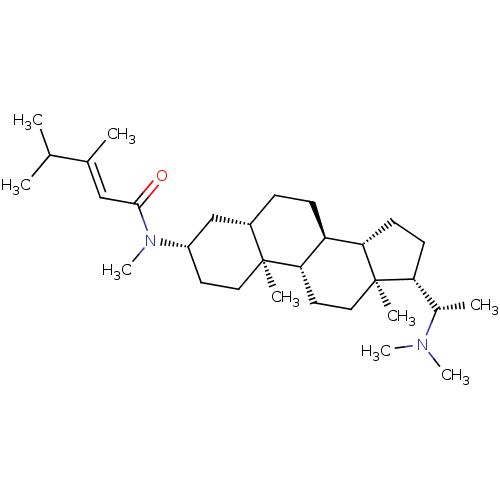 ((E)-3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135153 ((E)-3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135158 ((E)-2-Methyl-but-2-enoic acid [(3S,8S,9S,10R,13S,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135159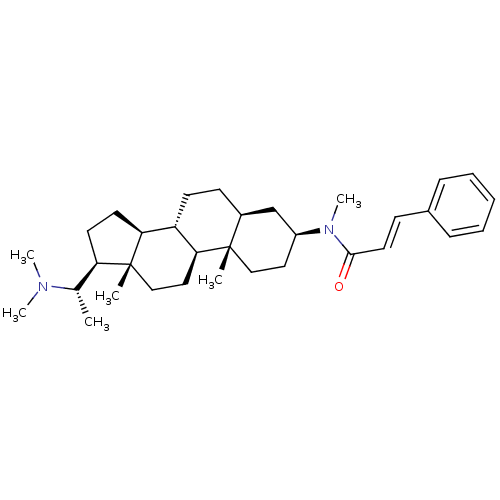 ((20S,2'E)-20-(N,N-dimethylamino)-3beta-(3'-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135152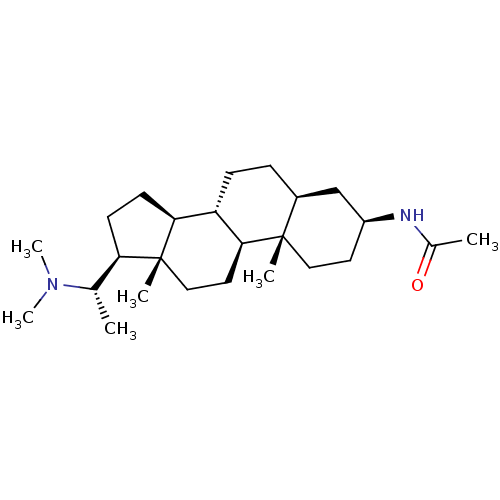 (CHEMBL422098 | N-[(3S,5S,8R,9S,10S,13S,14S,17S)-17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135150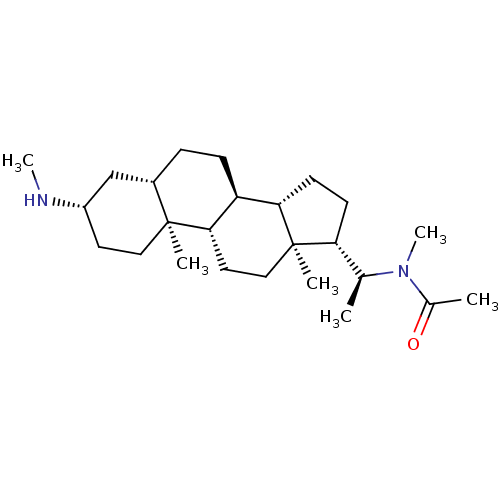 (CHEMBL147346 | N-Methyl-N-[1-((3S,5S,8R,9S,10S,13S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135155 (3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,10S,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135148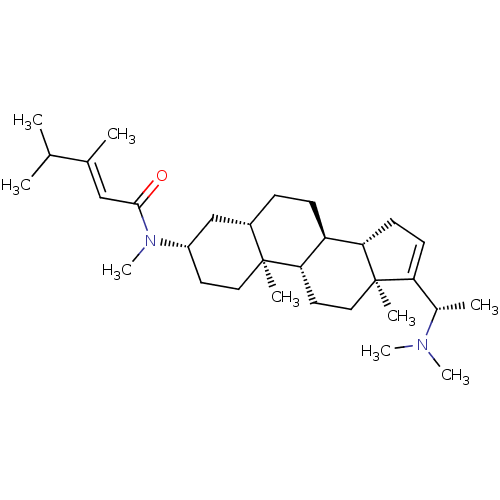 (1N-[14-(1-dimethylaminoethyl)-2,15-dimethyl-(1S,7S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135148 (1N-[14-(1-dimethylaminoethyl)-2,15-dimethyl-(1S,7S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135159 ((20S,2'E)-20-(N,N-dimethylamino)-3beta-(3'-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135151 (CHEMBL343365 | N-[(2S,3R,5S,8R,9S,10S,13S,14S,17S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135157 (Acetic acid (1S,6R,7R,10R,11S,12S,15S,16S)-17-((S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135149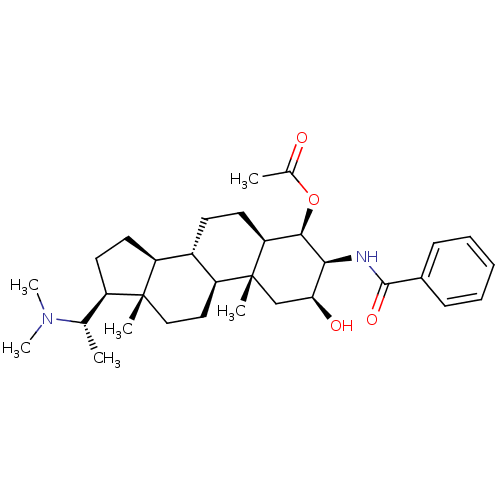 (Acetic acid (2S,3S,4R,5R,8S,9S,10R,13S,14S,17S)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50135156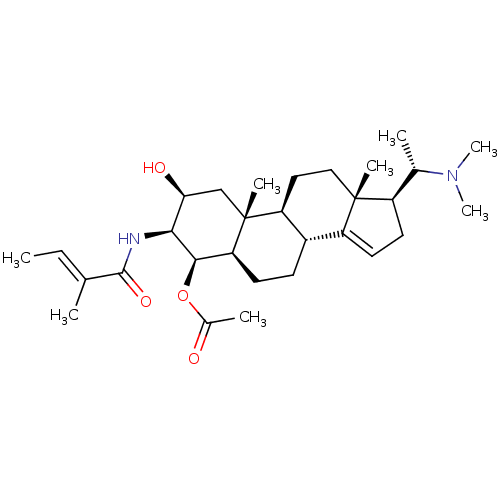 (Acetic acid (2S,3S,4R,5R,8R,9S,10R,13R,17S)-17-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against butyrylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135152 (CHEMBL422098 | N-[(3S,5S,8R,9S,10S,13S,14S,17S)-17...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135149 (Acetic acid (2S,3S,4R,5R,8S,9S,10R,13S,14S,17S)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135156 (Acetic acid (2S,3S,4R,5R,8R,9S,10R,13R,17S)-17-((S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50135150 (CHEMBL147346 | N-Methyl-N-[1-((3S,5S,8R,9S,10S,13S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity was determined against acetylcholinesterase from torpedo californica | J Med Chem 46: 5087-90 (2003) Article DOI: 10.1021/jm0309194 BindingDB Entry DOI: 10.7270/Q2DB818T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423170 (US10501440, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423180 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423185 (N-[(5R)-1-Amino-3-methyl-5H,6H,7H-cyclopenta[c]pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423169 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423183 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423179 (N-[(5R)-1-Amino-5H,6H,7H-cyclopenta[c]pyridin-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423195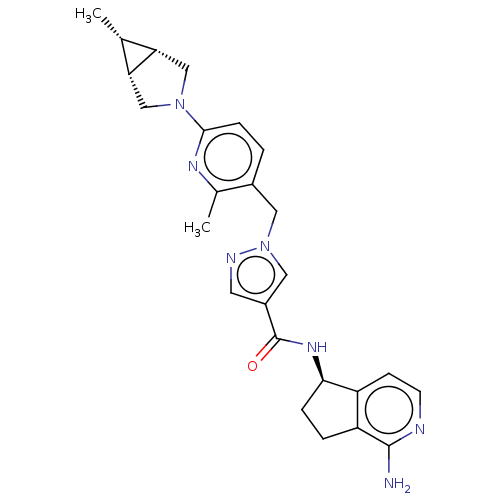 (1-[2-Methyl-6-((1S,5R,6R)-6-methyl-3-aza-bicyclo[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423171 (US10501440, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM423173 (US10501440, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human KLKB1 (0.01 U/mL; Enzyme Research Laboratories) or rat KLKB1 (0.625 nM; produced in-house) was incubated for 1 h at room temperature with 0.10 ... | US Patent US10501440 (2019) BindingDB Entry DOI: 10.7270/Q2QR50JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 550 total ) | Next | Last >> |