 Found 190 hits with Last Name = 'weller' and Initial = 'jm'
Found 190 hits with Last Name = 'weller' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233283

(CHEMBL4102262)Show SMILES OC(=O)c1ccc(Cl)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30ClF3N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233285

(CHEMBL4101413)Show SMILES Cc1cccc2ccc(nc12)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H]1CCc1ccccc1C(O)=O |r| Show InChI InChI=1S/C30H35N3O3/c1-20-6-4-9-23-14-15-27(32-28(20)23)33-18-16-24(17-19-33)29(34)31-26-11-5-8-22(26)13-12-21-7-2-3-10-25(21)30(35)36/h2-4,6-7,9-10,14-15,22,24,26H,5,8,11-13,16-19H2,1H3,(H,31,34)(H,35,36)/t22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233277

(CHEMBL4085873)Show SMILES Cc1ccc(C(O)=O)c(CC[C@@H]2CCC[C@@H]2NC(=O)C2CCN(CC2)c2ccc(cc2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C28H33F3N2O3/c1-18-5-12-24(27(35)36)21(17-18)7-6-19-3-2-4-25(19)32-26(34)20-13-15-33(16-14-20)23-10-8-22(9-11-23)28(29,30)31/h5,8-12,17,19-20,25H,2-4,6-7,13-16H2,1H3,(H,32,34)(H,35,36)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233281

(CHEMBL4084995)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C27H31F3N2O4/c28-27(29,30)36-22-12-10-21(11-13-22)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233278

(CHEMBL4064335)Show SMILES OC(=O)c1ccc(F)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233283

(CHEMBL4102262)Show SMILES OC(=O)c1ccc(Cl)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30ClF3N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233274
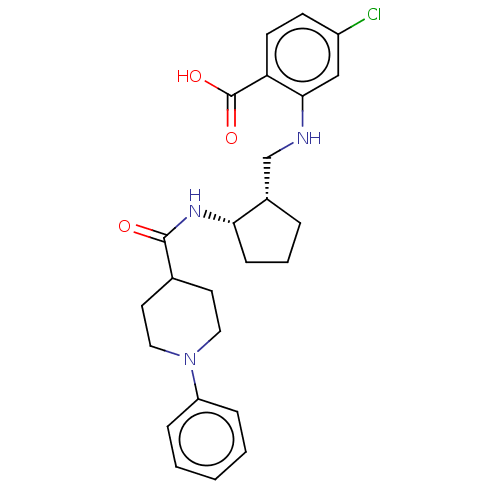
(CHEMBL4067045)Show SMILES OC(=O)c1ccc(Cl)cc1NC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H30ClN3O3/c26-19-9-10-21(25(31)32)23(15-19)27-16-18-5-4-8-22(18)28-24(30)17-11-13-29(14-12-17)20-6-2-1-3-7-20/h1-3,6-7,9-10,15,17-18,22,27H,4-5,8,11-14,16H2,(H,28,30)(H,31,32)/t18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233280

(CHEMBL4063350)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H30F3N3O3/c27-26(28,29)20-10-11-23(30-16-20)32-14-12-19(13-15-32)24(33)31-22-7-3-5-18(22)9-8-17-4-1-2-6-21(17)25(34)35/h1-2,4,6,10-11,16,18-19,22H,3,5,7-9,12-15H2,(H,31,33)(H,34,35)/t18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233276

(CHEMBL4078000)Show SMILES OC(=O)c1ccc(Cl)cc1OC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H28ClF3N2O4/c27-19-6-9-21(25(34)35)23(14-19)36-15-17-2-1-3-22(17)31-24(33)16-10-12-32(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22H,1-3,10-13,15H2,(H,31,33)(H,34,35)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233284
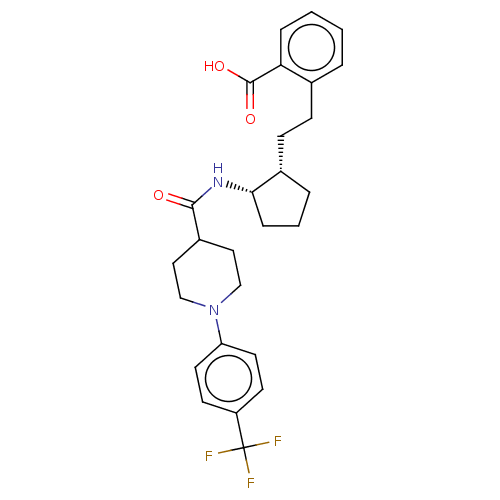
(CHEMBL4071976)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H31F3N2O3/c28-27(29,30)21-10-12-22(13-11-21)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233277

(CHEMBL4085873)Show SMILES Cc1ccc(C(O)=O)c(CC[C@@H]2CCC[C@@H]2NC(=O)C2CCN(CC2)c2ccc(cc2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C28H33F3N2O3/c1-18-5-12-24(27(35)36)21(17-18)7-6-19-3-2-4-25(19)32-26(34)20-13-15-33(16-14-20)23-10-8-22(9-11-23)28(29,30)31/h5,8-12,17,19-20,25H,2-4,6-7,13-16H2,1H3,(H,32,34)(H,35,36)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233275
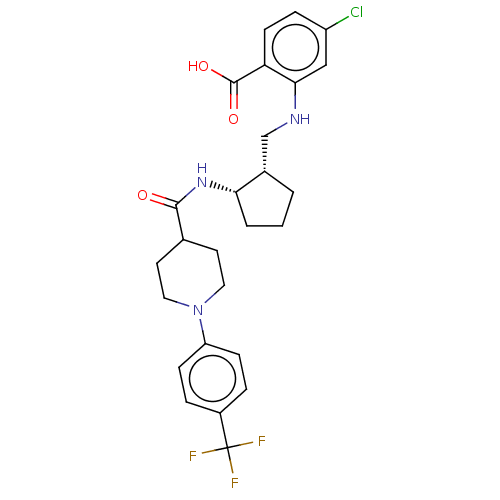
(CHEMBL4094518)Show SMILES OC(=O)c1ccc(Cl)cc1NC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H29ClF3N3O3/c27-19-6-9-21(25(35)36)23(14-19)31-15-17-2-1-3-22(17)32-24(34)16-10-12-33(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22,31H,1-3,10-13,15H2,(H,32,34)(H,35,36)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313
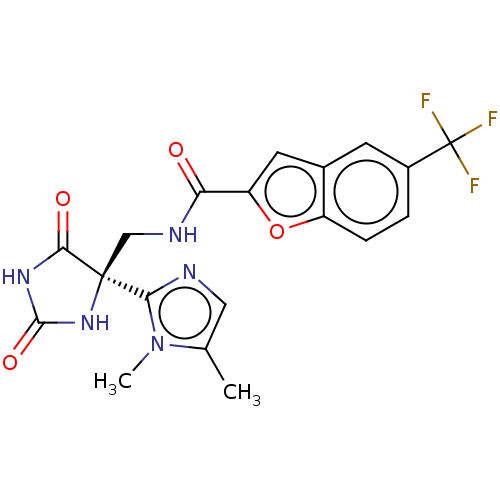
(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806
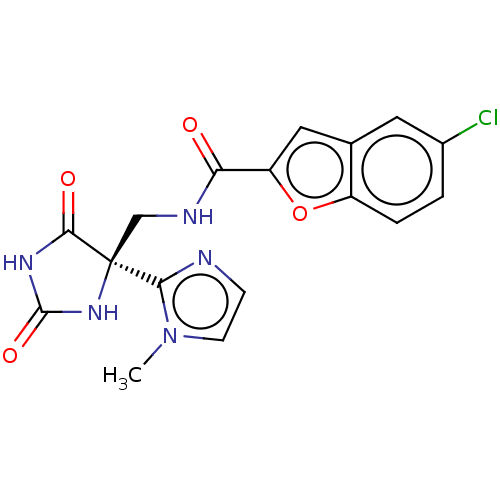
(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033806

(CHEMBL3358156)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C17H14ClN5O4/c1-23-5-4-19-14(23)17(15(25)21-16(26)22-17)8-20-13(24)12-7-9-6-10(18)2-3-11(9)27-12/h2-7H,8H2,1H3,(H,20,24)(H2,21,22,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233275

(CHEMBL4094518)Show SMILES OC(=O)c1ccc(Cl)cc1NC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H29ClF3N3O3/c27-19-6-9-21(25(35)36)23(14-19)31-15-17-2-1-3-22(17)32-24(34)16-10-12-33(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22,31H,1-3,10-13,15H2,(H,32,34)(H,35,36)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta-induced PGE2 production preincubated for 30 mins followed by IL-1beta addi... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233275

(CHEMBL4094518)Show SMILES OC(=O)c1ccc(Cl)cc1NC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H29ClF3N3O3/c27-19-6-9-21(25(35)36)23(14-19)31-15-17-2-1-3-22(17)32-24(34)16-10-12-33(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22,31H,1-3,10-13,15H2,(H,32,34)(H,35,36)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033808
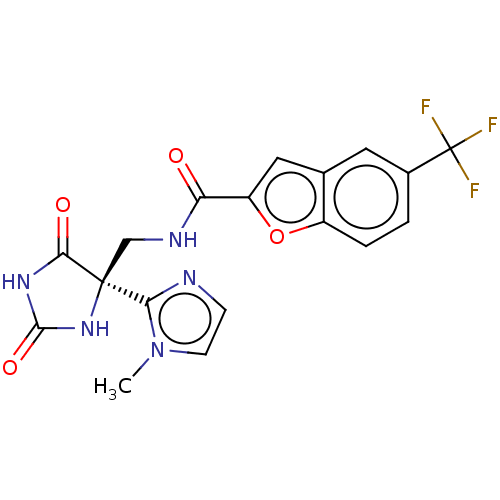
(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532312
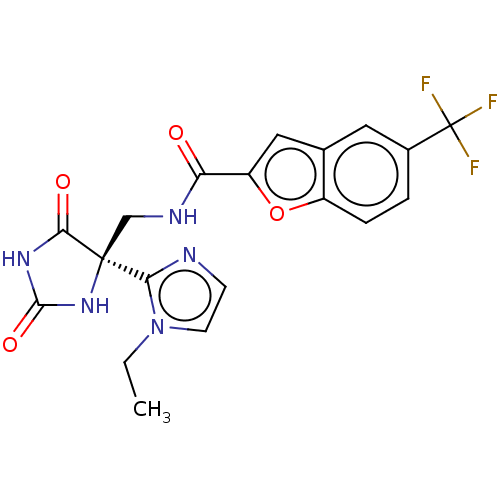
(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50033808

(CHEMBL3358158)Show SMILES Cn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H14F3N5O4/c1-26-5-4-22-14(26)17(15(28)24-16(29)25-17)8-23-13(27)12-7-9-6-10(18(19,20)21)2-3-11(9)30-12/h2-7H,8H2,1H3,(H,23,27)(H2,24,25,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532312

(CHEMBL4450729)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-2-27-6-5-23-15(27)18(16(29)25-17(30)26-18)9-24-14(28)13-8-10-7-11(19(20,21)22)3-4-12(10)31-13/h3-8H,2,9H2,1H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532313

(CHEMBL4436740)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(ccc3o2)C(F)(F)F)NC(=O)NC1=O |r| Show InChI InChI=1S/C19H16F3N5O4/c1-9-7-23-15(27(9)2)18(16(29)25-17(30)26-18)8-24-14(28)13-6-10-5-11(19(20,21)22)3-4-12(10)31-13/h3-7H,8H2,1-2H3,(H,24,28)(H2,25,26,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233284

(CHEMBL4071976)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H31F3N2O3/c28-27(29,30)21-10-12-22(13-11-21)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233279

(CHEMBL4092750)Show SMILES OC(=O)c1cccnc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H30F3N3O3/c27-26(28,29)19-7-9-20(10-8-19)32-15-12-18(13-16-32)24(33)31-22-5-1-3-17(22)6-11-23-21(25(34)35)4-2-14-30-23/h2,4,7-10,14,17-18,22H,1,3,5-6,11-13,15-16H2,(H,31,33)(H,34,35)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532311
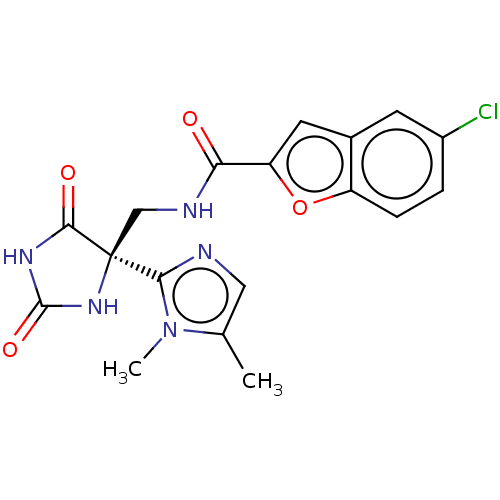
(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50238243

(CHEMBL4097165)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C17H14F3N3O4/c18-17(19,20)10-3-4-11-8(5-10)6-12(27-11)13(24)21-7-16(9-1-2-9)14(25)22-15(26)23-16/h3-6,9H,1-2,7H2,(H,21,24)(H2,22,23,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233278

(CHEMBL4064335)Show SMILES OC(=O)c1ccc(F)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233285

(CHEMBL4101413)Show SMILES Cc1cccc2ccc(nc12)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H]1CCc1ccccc1C(O)=O |r| Show InChI InChI=1S/C30H35N3O3/c1-20-6-4-9-23-14-15-27(32-28(20)23)33-18-16-24(17-19-33)29(34)31-26-11-5-8-22(26)13-12-21-7-2-3-10-25(21)30(35)36/h2-4,6-7,9-10,14-15,22,24,26H,5,8,11-13,16-19H2,1H3,(H,31,34)(H,35,36)/t22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532311

(CHEMBL4587825)Show SMILES Cc1cnc(n1C)[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-9-7-20-15(24(9)2)18(16(26)22-17(27)23-18)8-21-14(25)13-6-10-5-11(19)3-4-12(10)28-13/h3-7H,8H2,1-2H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532310
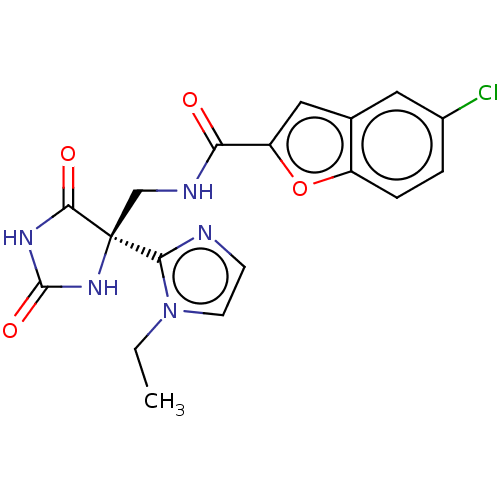
(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233276

(CHEMBL4078000)Show SMILES OC(=O)c1ccc(Cl)cc1OC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H28ClF3N2O4/c27-19-6-9-21(25(34)35)23(14-19)36-15-17-2-1-3-22(17)31-24(33)16-10-12-32(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22H,1-3,10-13,15H2,(H,31,33)(H,34,35)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033805
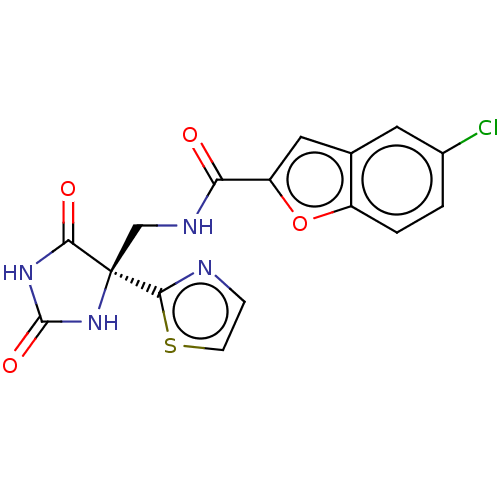
(CHEMBL3358155)Show SMILES Clc1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)c1nccs1 |r| Show InChI InChI=1S/C16H11ClN4O4S/c17-9-1-2-10-8(5-9)6-11(25-10)12(22)19-7-16(14-18-3-4-26-14)13(23)20-15(24)21-16/h1-6H,7H2,(H,19,22)(H2,20,21,23,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50033805

(CHEMBL3358155)Show SMILES Clc1ccc2oc(cc2c1)C(=O)NC[C@]1(NC(=O)NC1=O)c1nccs1 |r| Show InChI InChI=1S/C16H11ClN4O4S/c17-9-1-2-10-8(5-9)6-11(25-10)12(22)19-7-16(14-18-3-4-26-14)13(23)20-15(24)21-16/h1-6H,7H2,(H,19,22)(H2,20,21,23,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50532310

(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532310

(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50532310

(CHEMBL4589438)Show SMILES CCn1ccnc1[C@]1(CNC(=O)c2cc3cc(Cl)ccc3o2)NC(=O)NC1=O |r| Show InChI InChI=1S/C18H16ClN5O4/c1-2-24-6-5-20-15(24)18(16(26)22-17(27)23-18)9-21-14(25)13-8-10-7-11(19)3-4-12(10)28-13/h3-8H,2,9H2,1H3,(H,21,25)(H2,22,23,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using 43-mer VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG as substrate measured after 3 hrs by AlphaScreen assay |
J Med Chem 59: 5810-22 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00398
BindingDB Entry DOI: 10.7270/Q280563T |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233281

(CHEMBL4084995)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C27H31F3N2O4/c28-27(29,30)36-22-12-10-21(11-13-22)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data