 Found 289 hits with Last Name = 'weng' and Initial = 'l'
Found 289 hits with Last Name = 'weng' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595147

(CHEMBL5205840)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595148

(CHEMBL5202600)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1cnccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595161

(CHEMBL5172072)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595158
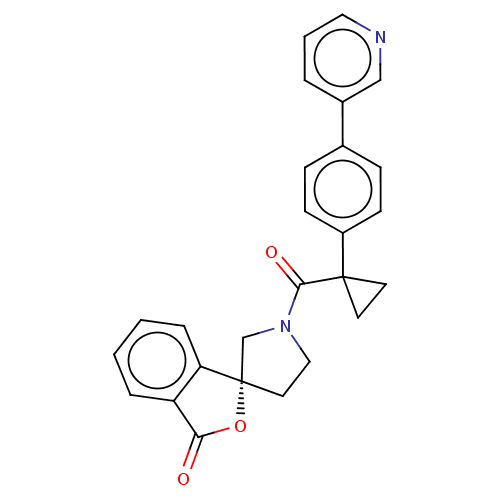
(CHEMBL5203601)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595152

(CHEMBL5187458)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(nc1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595147

(CHEMBL5205840)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human C-src using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595159

(CHEMBL5175978)Show SMILES CNC(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595157

(CHEMBL5188613)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1ccccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595161

(CHEMBL5172072)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595159

(CHEMBL5175978)Show SMILES CNC(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595144

(CHEMBL5183831)Show SMILES Cc1cccc(C)c1C1CCN(C1)C(=O)C1(CC1)c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595156
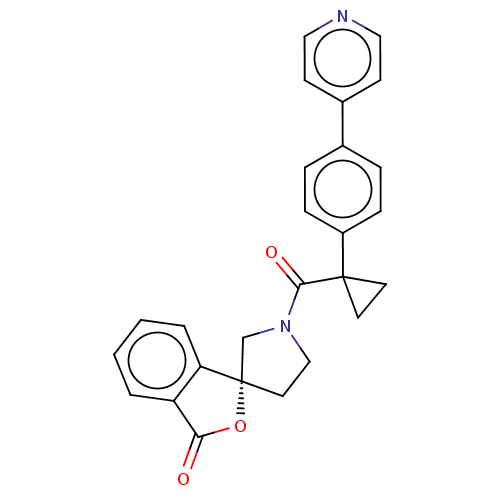
(CHEMBL5187680)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1ccncc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595162

(CHEMBL5187093)Show SMILES CN(C)C(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595160

(CHEMBL5194632)Show SMILES CN(C)C(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595158

(CHEMBL5203601)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203526
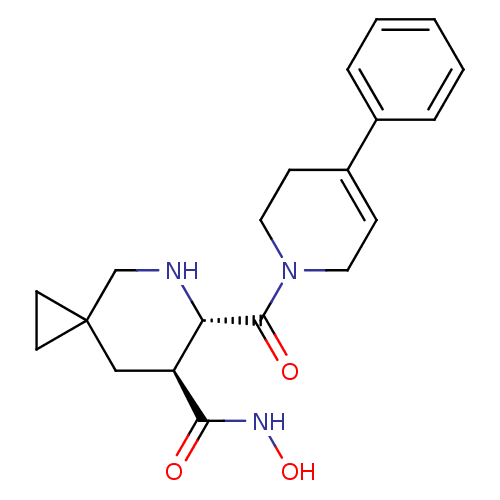
((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595160

(CHEMBL5194632)Show SMILES CN(C)C(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589350

(CHEMBL5192382)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CCC2(C1)OC(=O)c1ccccc21 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595141
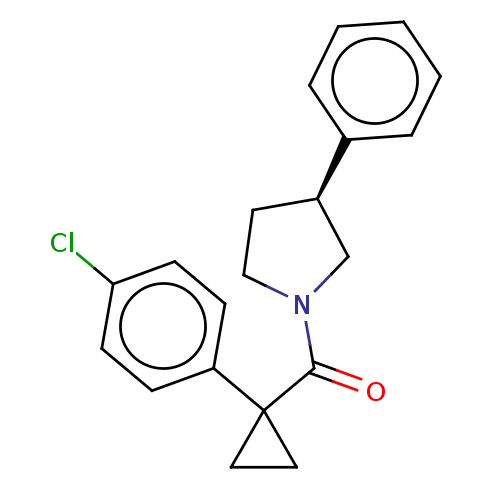
(CHEMBL5201898)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@H](C1)c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595136

(CHEMBL5177277) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239401

(3-Adamantan-1-yl-6,7,8,9-tetrahydro-5H-[1,2,4]tria...)Show SMILES C1C2CC3CC1CC(C2)(C3)c1nnc2CCCCCn12 |TLB:4:3:0.5.6:8,0:5:1.8.2:9,0:1:5.6.4:9,THB:4:5:8:2.3.9| Show InChI InChI=1S/C17H25N3/c1-2-4-15-18-19-16(20(15)5-3-1)17-9-12-6-13(10-17)8-14(7-12)11-17/h12-14H,1-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595162

(CHEMBL5187093)Show SMILES CN(C)C(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595143

(CHEMBL5183130) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595157

(CHEMBL5188613)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1ccccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595148

(CHEMBL5202600)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1cnccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595154
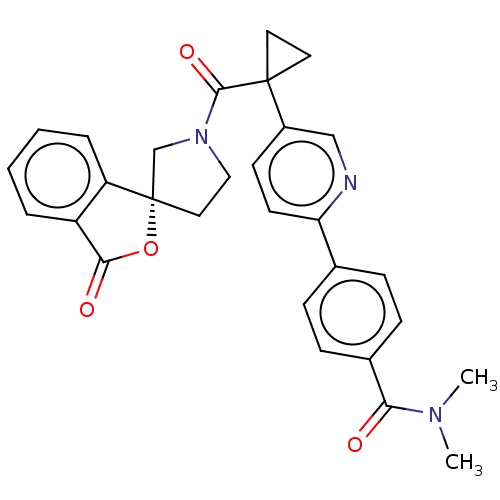
(CHEMBL5173694)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc(cn1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595154

(CHEMBL5173694)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc(cn1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246861
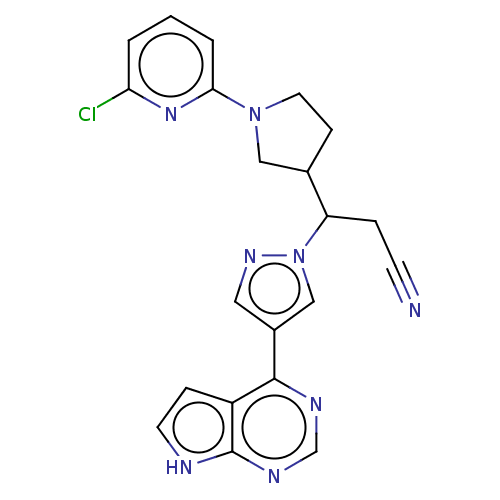
(3-[1-(6- chloropyridin-2- yl)pyrrolidin-3-yl]-3- [...)Show SMILES Clc1cccc(n1)N1CCC(C1)C(CC#N)n1cc(cn1)-c1ncnc2[nH]ccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246864
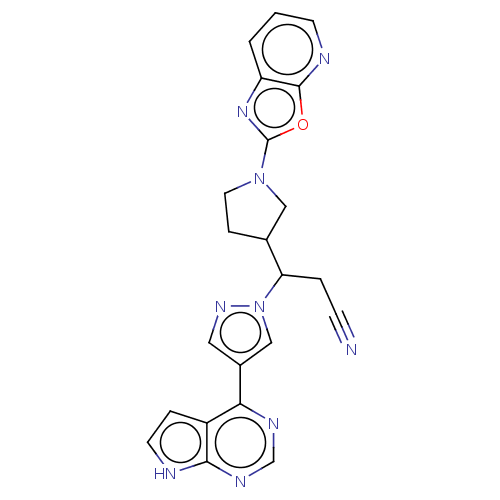
(3-(1- [1,3]oxazolo[5,4- b]pyridin-2- ylpyrrolidin-...)Show SMILES N#CCC(C1CCN(C1)c1nc2cccnc2o1)n1cc(cn1)-c1ncnc2[nH]ccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246866
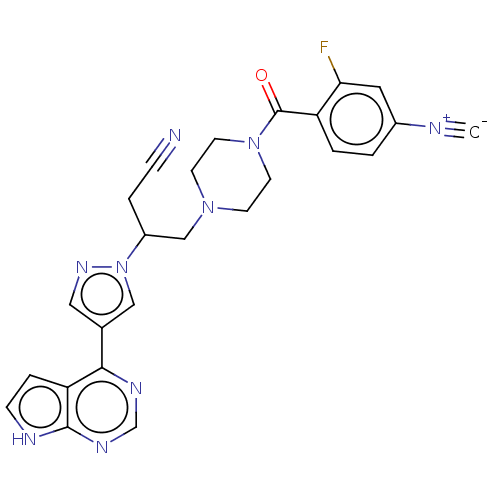
(4-[(4-{3-cyano-2-[4- (7H-pyrrolo[2,3- d]pyrimidin-...)Show SMILES Fc1cc(ccc1C(=O)N1CCN(CC(CC#N)n2cc(cn2)-c2ncnc3[nH]ccc23)CC1)[N+]#[C-] | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246867
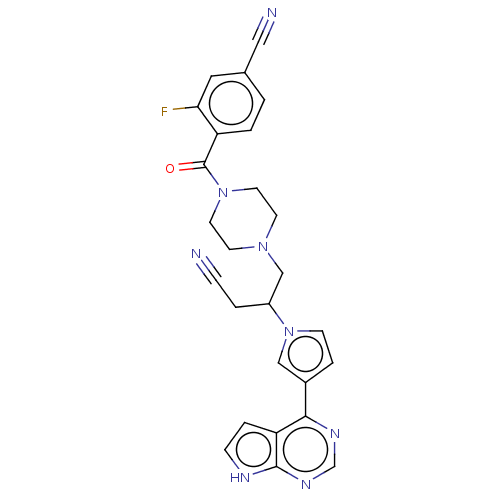
(4-[(4-{3-cyano-2-[3- (7H-pyrrolo[2,3- d]pyrimidin-...)Show SMILES Fc1cc(ccc1C(=O)N1CCN(CC(CC#N)n2ccc(c2)-c2ncnc3[nH]ccc23)CC1)C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246868

(US10053465, 7 | US10065963, Compound 7 | US1012515...)Show SMILES Fc1c(ccnc1C(F)(F)F)C(=O)N1CCC(CC1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C26H23F4N9O/c27-20-18(1-7-32-22(20)26(28,29)30)24(40)37-9-3-17(4-10-37)38-13-25(14-38,5-6-31)39-12-16(11-36-39)21-19-2-8-33-23(19)35-15-34-21/h1-2,7-8,11-12,15,17H,3-5,9-10,13-14H2,(H,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
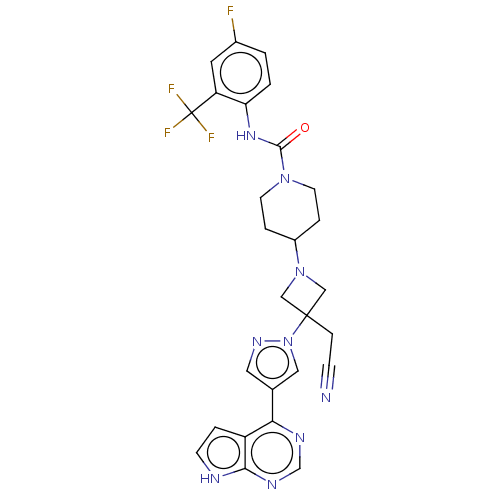
(Homo sapiens (Human)) | BDBM246869
(4-{3-(Cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES Fc1ccc(NC(=O)N2CCC(CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)c(c1)C(F)(F)F Show InChI InChI=1S/C27H25F4N9O/c28-18-1-2-22(21(11-18)27(29,30)31)37-25(41)38-9-4-19(5-10-38)39-14-26(15-39,6-7-32)40-13-17(12-36-40)23-20-3-8-33-24(20)35-16-34-23/h1-3,8,11-13,16,19H,4-6,9-10,14-15H2,(H,37,41)(H,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
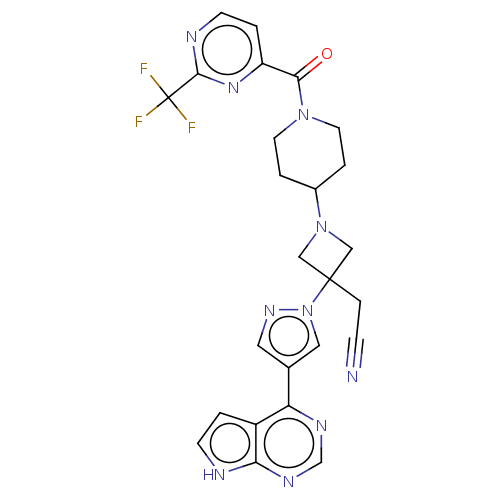
(Homo sapiens (Human)) | BDBM246870
(US10053465, 9 | US10065963, Compound 9 | US1012515...)Show SMILES FC(F)(F)c1nccc(n1)C(=O)N1CCC(CC1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C25H23F3N10O/c26-25(27,28)23-31-8-2-19(35-23)22(39)36-9-3-17(4-10-36)37-13-24(14-37,5-6-29)38-12-16(11-34-38)20-18-1-7-30-21(18)33-15-32-20/h1-2,7-8,11-12,15,17H,3-5,9-10,13-14H2,(H,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246871
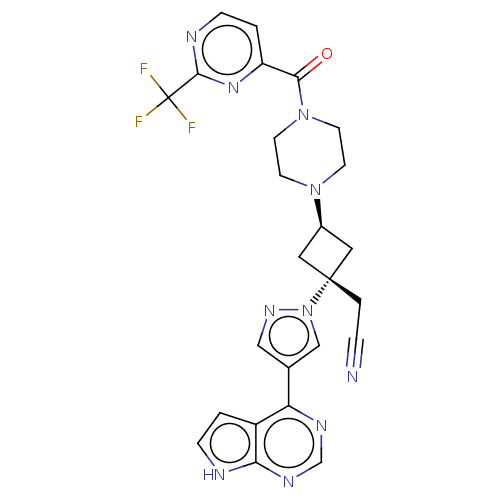
(US10053465, 10 | US10065963, Compound 10 | US10125...)Show SMILES FC(F)(F)c1nccc(n1)C(=O)N1CCN(CC1)[C@H]1C[C@@](CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r,wU:20.27,wD:18.19,(-2.27,10.39,;-.79,10,;.3,11.08,;-.79,11.54,;-.39,8.51,;1.1,8.11,;1.5,6.62,;.41,5.53,;-1.08,5.93,;-1.48,7.42,;-2.17,4.84,;-3.66,5.24,;-1.77,3.35,;-2.86,2.27,;-2.46,.78,;-.97,.38,;.12,1.47,;-.28,2.96,;-.57,-1.11,;-1.34,-2.44,;-.01,-3.21,;1.48,-3.61,;2.57,-2.52,;3.66,-1.43,;.76,-1.88,;-.78,-4.55,;-.3,-6.01,;-1.55,-6.92,;-2.8,-6.01,;-2.32,-4.55,;-1.55,-8.46,;-2.88,-9.23,;-2.88,-10.77,;-1.55,-11.54,;-.22,-10.77,;1.25,-11.24,;2.15,-10,;1.25,-8.75,;-.22,-9.23,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246872
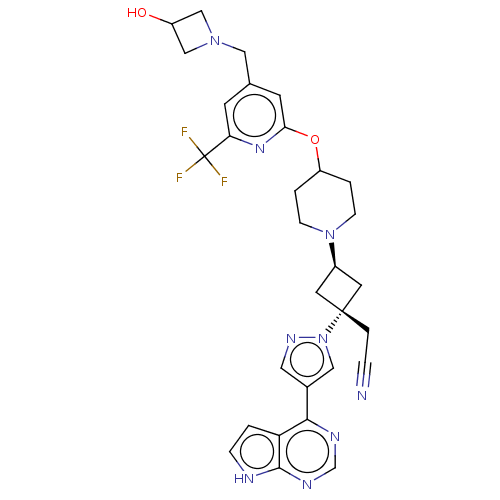
(US10053465, 11 | US10065963, Compound 11 | US10125...)Show SMILES OC1CN(Cc2cc(OC3CCN(CC3)[C@H]3C[C@@](CC#N)(C3)n3cc(cn3)-c3ncnc4[nH]ccc34)nc(c2)C(F)(F)F)C1 |r,wU:17.23,wD:15.15,(2.14,12.62,;1.05,11.53,;1.05,9.99,;-.49,9.99,;-1.58,8.91,;-1.18,7.42,;-2.27,6.33,;-1.87,4.84,;-2.96,3.75,;-2.56,2.27,;-3.65,1.18,;-3.25,-.31,;-1.77,-.71,;-.68,.38,;-1.08,1.87,;-1.37,-2.2,;-2.14,-3.53,;-.8,-4.3,;.68,-4.7,;1.77,-3.61,;2.86,-2.52,;-.03,-2.97,;-1.57,-5.63,;-1.1,-7.1,;-2.34,-8,;-3.59,-7.1,;-3.11,-5.63,;-2.34,-9.54,;-3.68,-10.31,;-3.68,-11.85,;-2.34,-12.62,;-1.01,-11.85,;.45,-12.33,;1.36,-11.08,;.45,-9.84,;-1.01,-10.31,;-.39,4.44,;.7,5.53,;.3,7.02,;2.19,5.13,;3.28,6.22,;2.59,3.65,;3.68,4.74,;-.49,11.53,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246873
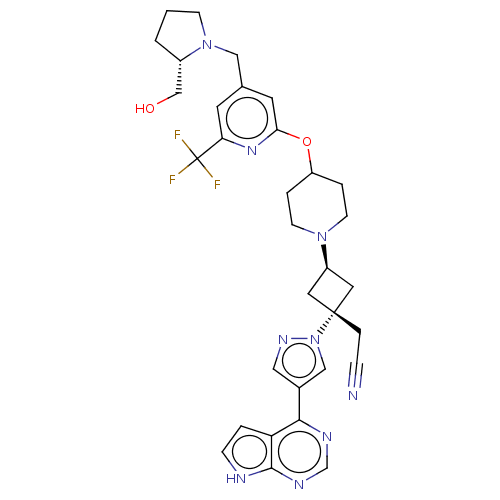
(US10053465, 12 | US10065963, Compound 12 | US10125...)Show SMILES OC[C@@H]1CCCN1Cc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:20.27,2.1,wD:18.19,(3.36,8.8,;1.82,8.8,;1.05,10.14,;1.52,11.59,;.29,12.48,;-.95,11.59,;-.49,10.14,;-1.58,9.05,;-1.18,7.56,;-2.27,6.47,;-1.87,4.98,;-2.96,3.9,;-2.56,2.41,;-3.65,1.32,;-3.25,-.17,;-1.77,-.57,;-.68,.52,;-1.08,2.01,;-1.37,-2.05,;-2.14,-3.39,;-.8,-4.16,;.68,-4.56,;1.77,-3.47,;2.86,-2.38,;-.03,-2.82,;-1.57,-5.49,;-1.1,-6.96,;-2.34,-7.86,;-3.59,-6.96,;-3.11,-5.49,;-2.34,-9.4,;-3.68,-10.17,;-3.68,-11.71,;-2.34,-12.48,;-1.01,-11.71,;.45,-12.19,;1.36,-10.94,;.45,-9.7,;-1.01,-10.17,;-.39,4.59,;.7,5.67,;.3,7.16,;2.19,5.28,;3.28,6.37,;2.59,3.79,;3.68,4.88,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246874
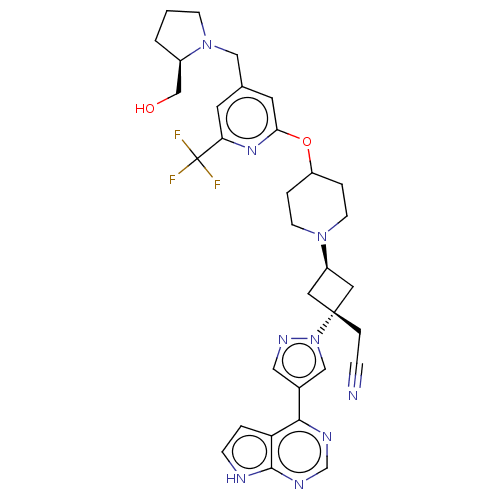
(US10053465, 13 | US10065963, Compound 13 | US10125...)Show SMILES OC[C@H]1CCCN1Cc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:20.27,wD:18.19,2.1,(3.36,8.8,;1.82,8.8,;1.05,10.14,;1.52,11.59,;.29,12.48,;-.95,11.59,;-.49,10.14,;-1.58,9.05,;-1.18,7.56,;-2.27,6.47,;-1.87,4.98,;-2.96,3.9,;-2.56,2.41,;-3.65,1.32,;-3.25,-.17,;-1.77,-.57,;-.68,.52,;-1.08,2.01,;-1.37,-2.05,;-2.14,-3.39,;-.8,-4.16,;.68,-4.56,;1.77,-3.47,;2.86,-2.38,;-.03,-2.82,;-1.57,-5.49,;-1.1,-6.96,;-2.34,-7.86,;-3.59,-6.96,;-3.11,-5.49,;-2.34,-9.4,;-3.68,-10.17,;-3.68,-11.71,;-2.34,-12.48,;-1.01,-11.71,;.45,-12.19,;1.36,-10.94,;.45,-9.7,;-1.01,-10.17,;-.39,4.59,;.7,5.67,;.3,7.16,;2.19,5.28,;3.28,6.37,;2.59,3.79,;3.68,4.88,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246875
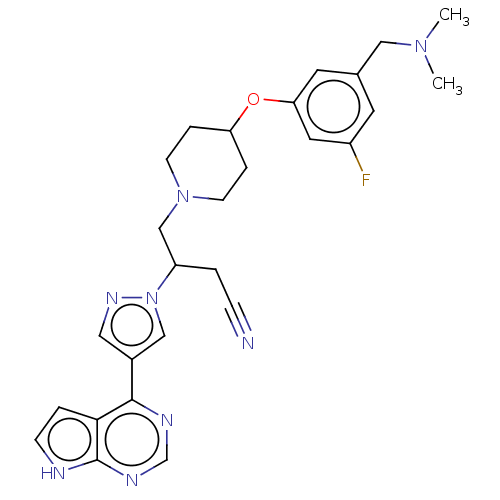
(4-(4-{3- [(dimethylamino) methyl]-5- fluorophenoxy...)Show SMILES CN(C)Cc1cc(F)cc(OC2CCN(CC(CC#N)n3cc(cn3)-c3ncnc4[nH]ccc34)CC2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246876
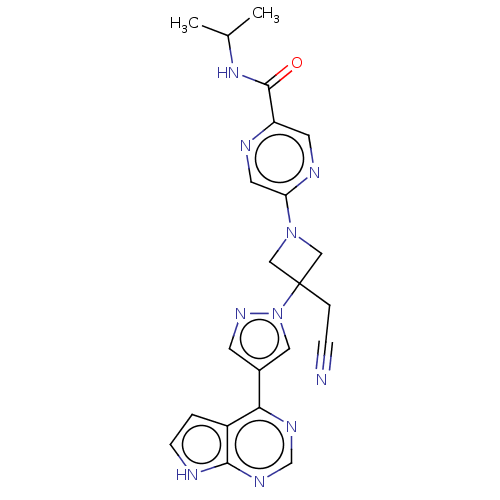
(5-{3-(cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES CC(C)NC(=O)c1cnc(cn1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C22H22N10O/c1-14(2)30-21(33)17-8-26-18(9-25-17)31-11-22(12-31,4-5-23)32-10-15(7-29-32)19-16-3-6-24-20(16)28-13-27-19/h3,6-10,13-14H,4,11-12H2,1-2H3,(H,30,33)(H,24,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
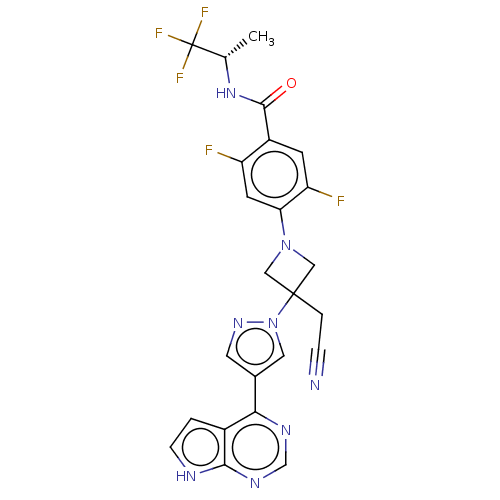
(Homo sapiens (Human)) | BDBM246877
(4-{3-(cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES C[C@H](NC(=O)c1cc(F)c(cc1F)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
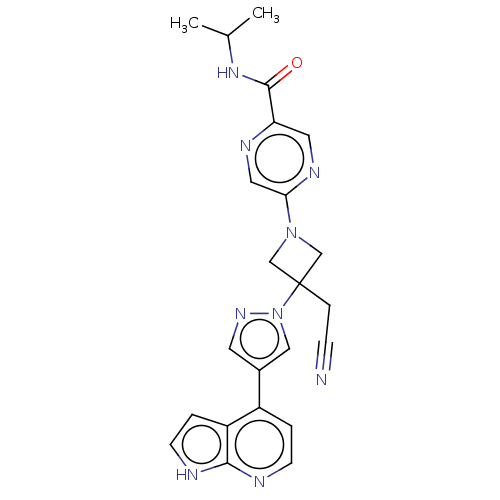
(Homo sapiens (Human)) | BDBM262166
(5-{3-(cyanomethyl)- 3-[4-(1H-pyrrolo[2,3- b]pyridi...)Show SMILES CC(C)NC(=O)c1cnc(cn1)N1CC(CC#N)(C1)n1cc(cn1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C23H23N9O/c1-15(2)30-22(33)19-10-28-20(11-27-19)31-13-23(14-31,5-6-24)32-12-16(9-29-32)17-3-7-25-21-18(17)4-8-26-21/h3-4,7-12,15H,5,13-14H2,1-2H3,(H,25,26)(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246879

(US10053465, 18 | US10065963, Compound 18 | US10125...)Show SMILES OCCc1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(n1)C(F)(F)F |r,wU:10.13,7.6,(9.15,6.25,;7.82,5.48,;6.48,6.25,;5.15,5.48,;3.82,6.25,;2.48,5.48,;1.15,6.25,;-.18,5.48,;-.18,3.94,;-1.52,3.17,;-2.85,3.94,;-2.85,5.48,;-1.52,6.25,;-4.19,3.17,;-4.58,1.68,;-6.07,2.08,;-7.61,2.08,;-8.38,3.41,;-9.15,4.74,;-5.67,3.56,;-6.84,.74,;-6.37,-.72,;-7.61,-1.63,;-8.86,-.72,;-8.38,.74,;-7.61,-3.17,;-8.95,-3.94,;-8.95,-5.48,;-7.61,-6.25,;-6.28,-5.48,;-4.81,-5.95,;-3.91,-4.71,;-4.81,-3.46,;-6.28,-3.94,;2.48,3.94,;3.82,3.17,;5.15,3.94,;3.82,1.63,;5.15,.86,;2.48,.86,;3.82,.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246880

(US10053465, 19 | US10065963, Compound 19 | US10125...)Show SMILES CCNCc1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:11.14,8.7,(9.82,5.48,;8.48,6.25,;7.15,5.48,;5.82,6.25,;4.48,5.48,;3.15,6.25,;1.82,5.48,;.48,6.25,;-.85,5.48,;-.85,3.94,;-2.18,3.17,;-3.52,3.94,;-3.52,5.48,;-2.18,6.25,;-4.85,3.17,;-5.25,1.68,;-6.74,2.08,;-8.28,2.08,;-9.05,3.41,;-9.82,4.74,;-6.34,3.56,;-7.51,.74,;-7.03,-.72,;-8.28,-1.63,;-9.52,-.72,;-9.05,.74,;-8.28,-3.17,;-9.61,-3.94,;-9.61,-5.48,;-8.28,-6.25,;-6.94,-5.48,;-5.48,-5.95,;-4.57,-4.71,;-5.48,-3.46,;-6.94,-3.94,;1.82,3.94,;3.15,3.17,;4.48,3.94,;3.15,1.63,;4.48,.86,;1.82,.86,;3.15,.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
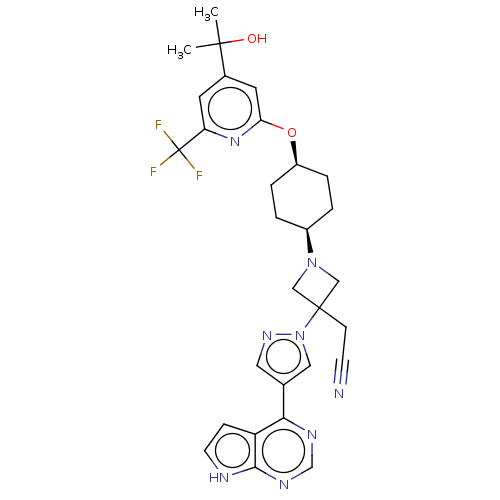
(Homo sapiens (Human)) | BDBM246881
(US10053465, 20 | US10065963, Compound 20 | US10125...)Show SMILES CC(C)(O)c1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:11.14,8.7,(8.48,4.71,;7.15,5.48,;7.15,7.02,;8.48,6.25,;5.82,4.71,;4.48,5.48,;3.15,4.71,;1.82,5.48,;.48,4.71,;.48,3.17,;-.85,2.4,;-2.18,3.17,;-2.18,4.71,;-.85,5.48,;-3.52,2.4,;-3.92,.91,;-5.4,1.31,;-6.94,1.31,;-7.71,2.64,;-8.48,3.97,;-5.01,2.79,;-6.17,-.03,;-5.7,-1.49,;-6.94,-2.4,;-8.19,-1.49,;-7.71,-.03,;-6.94,-3.94,;-8.28,-4.71,;-8.28,-6.25,;-6.94,-7.02,;-5.61,-6.25,;-4.15,-6.72,;-3.24,-5.48,;-4.15,-4.23,;-5.61,-4.71,;3.15,3.17,;4.48,2.4,;5.82,3.17,;4.48,.86,;5.82,.09,;3.15,.09,;4.48,-.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Assays are carried out at room temperature in 50 mM HEPES, pH 7.4, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT and CHAPS 0.04%. Reactions are initiated by the a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63R3N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data