 Found 254 hits with Last Name = 'wilson' and Initial = 'z'
Found 254 hits with Last Name = 'wilson' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052
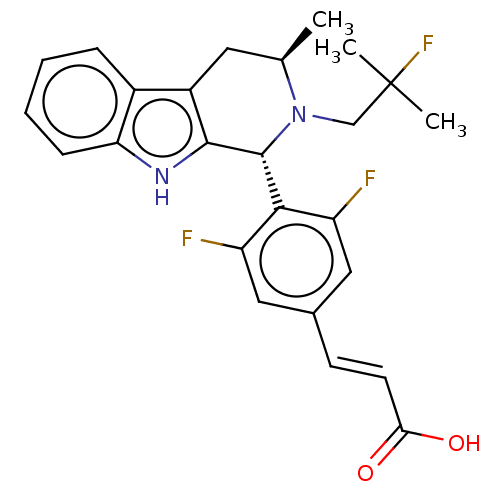
(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
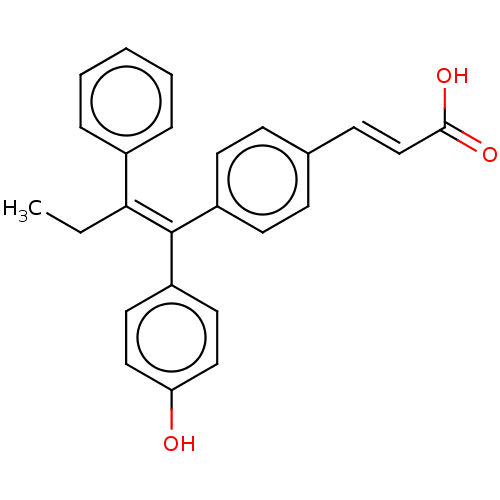
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
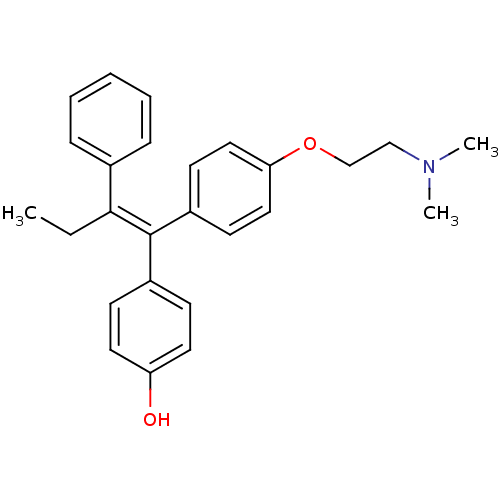
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
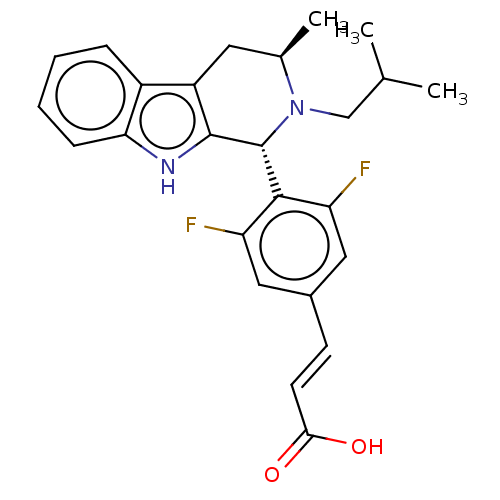
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468005
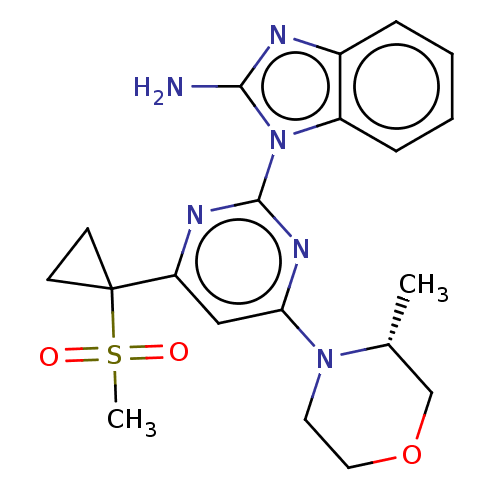
(CHEMBL4288033)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-n1c(N)nc2ccccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H24N6O3S/c1-13-12-29-10-9-25(13)17-11-16(20(7-8-20)30(2,27)28)23-19(24-17)26-15-6-4-3-5-14(15)22-18(26)21/h3-6,11,13H,7-10,12H2,1-2H3,(H2,21,22)/t13-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468002

(CHEMBL4291436)Show SMILES CNc1nc2ccccc2n1-c1nc(cc(n1)C1(CC1)S(C)(=O)=O)N1CCOC[C@H]1C |r| Show InChI InChI=1S/C21H26N6O3S/c1-14-13-30-11-10-26(14)18-12-17(21(8-9-21)31(3,28)29)24-20(25-18)27-16-7-5-4-6-15(16)23-19(27)22-2/h4-7,12,14H,8-11,13H2,1-3H3,(H,22,23)/t14-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468009

(CHEMBL4286105)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-n1c(N)nc2cc(F)ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23FN6O3S/c1-12-11-30-8-7-26(12)17-10-16(20(5-6-20)31(2,28)29)24-19(25-17)27-15-4-3-13(21)9-14(15)23-18(27)22/h3-4,9-10,12H,5-8,11H2,1-2H3,(H2,22,23)/t12-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468003

(CHEMBL4281240)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cncc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23N5O3S/c1-13-12-28-8-7-25(13)18-9-17(20(4-5-20)29(2,26)27)23-19(24-18)15-10-21-11-16-14(15)3-6-22-16/h3,6,9-11,13,22H,4-5,7-8,12H2,1-2H3/t13-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468017
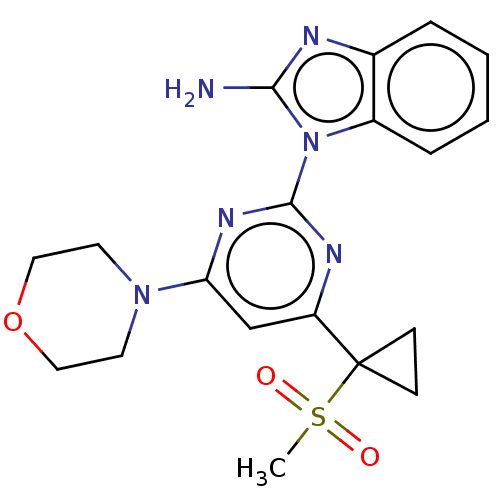
(CHEMBL4291783)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-n1c(N)nc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C19H22N6O3S/c1-29(26,27)19(6-7-19)15-12-16(24-8-10-28-11-9-24)23-18(22-15)25-14-5-3-2-4-13(14)21-17(25)20/h2-5,12H,6-11H2,1H3,(H2,20,21) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50041611
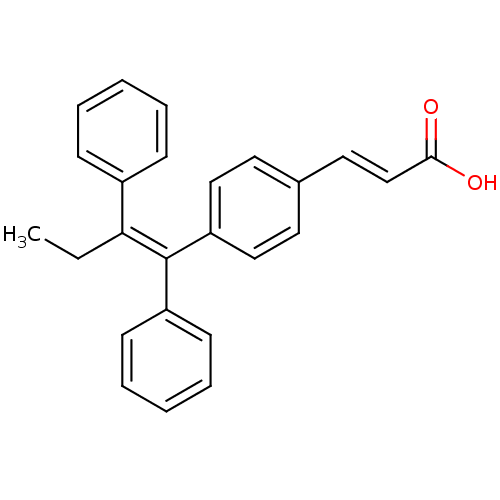
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM60432

(BDBM50468001 | US11236089, Compound AZD-6738 | US8...)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1ccnc2[nH]ccc12)C1(CC1)[S@](C)(=N)=O |r| Show InChI InChI=1S/C20H24N6O2S/c1-13-12-28-10-9-26(13)17-11-16(20(5-6-20)29(2,21)27)24-19(25-17)15-4-8-23-18-14(15)3-7-22-18/h3-4,7-8,11,13,21H,5-6,9-10,12H2,1-2H3,(H,22,23)/t13-,29-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427326
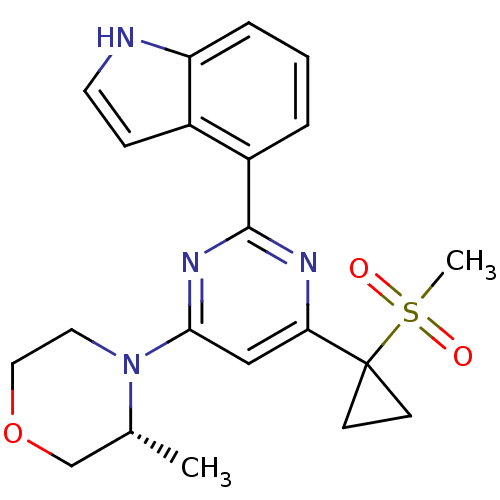
(CHEMBL2325697)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cccc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14-13-28-11-10-25(14)19-12-18(21(7-8-21)29(2,26)27)23-20(24-19)16-4-3-5-17-15(16)6-9-22-17/h3-6,9,12,14,22H,7-8,10-11,13H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468021

(CHEMBL4278556)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cncc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C19H21N5O3S/c1-28(25,26)19(3-4-19)16-10-17(24-6-8-27-9-7-24)23-18(22-16)14-11-20-12-15-13(14)2-5-21-15/h2,5,10-12,21H,3-4,6-9H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468004
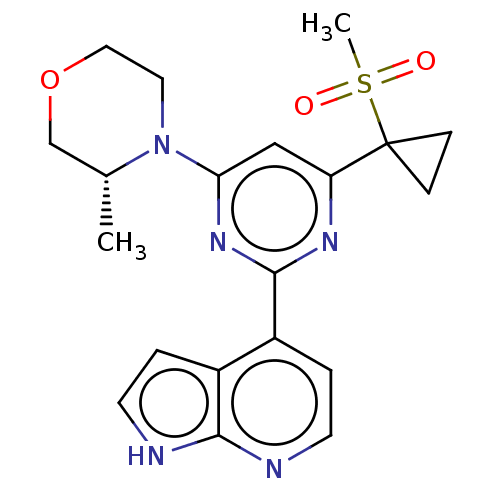
(CHEMBL4286472)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1ccnc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23N5O3S/c1-13-12-28-10-9-25(13)17-11-16(20(5-6-20)29(2,26)27)23-19(24-17)15-4-8-22-18-14(15)3-7-21-18/h3-4,7-8,11,13H,5-6,9-10,12H2,1-2H3,(H,21,22)/t13-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM103467
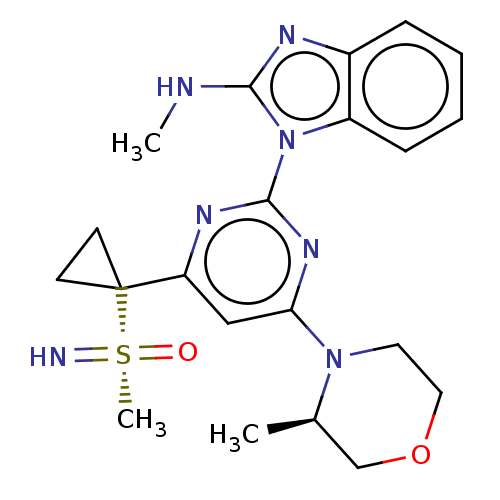
(US8552004, 2.03)Show SMILES CNc1nc2ccccc2n1-c1nc(cc(n1)C1(CC1)[S@](C)(=N)=O)N1CCOC[C@H]1C |r| Show InChI InChI=1S/C21H27N7O2S/c1-14-13-30-11-10-27(14)18-12-17(21(8-9-21)31(3,22)29)25-20(26-18)28-16-7-5-4-6-15(16)24-19(28)23-2/h4-7,12,14H,3,8-11,13H2,1-2H3,(H2,22,29)(H,23,24)/t14-,31?/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514979
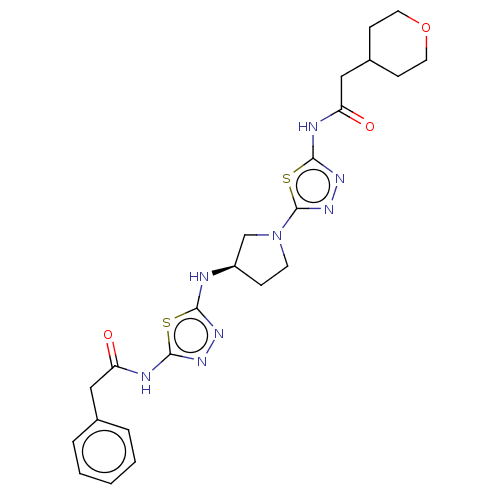
(CHEMBL4457936)Show SMILES O=C(CC1CCOCC1)Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C23H28N8O3S2/c32-18(12-15-4-2-1-3-5-15)25-21-28-27-20(35-21)24-17-6-9-31(14-17)23-30-29-22(36-23)26-19(33)13-16-7-10-34-11-8-16/h1-5,16-17H,6-14H2,(H,24,27)(H,25,28,32)(H,26,29,33)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427327

(CHEMBL2325699)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CC2CCC(C1)O2 |TLB:9:22:29:25.26| Show InChI InChI=1S/C22H24N4O3S/c1-30(27,28)22(8-9-22)19-11-20(26-12-14-5-6-15(13-26)29-14)25-21(24-19)17-3-2-4-18-16(17)7-10-23-18/h2-4,7,10-11,14-15,23H,5-6,8-9,12-13H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514986
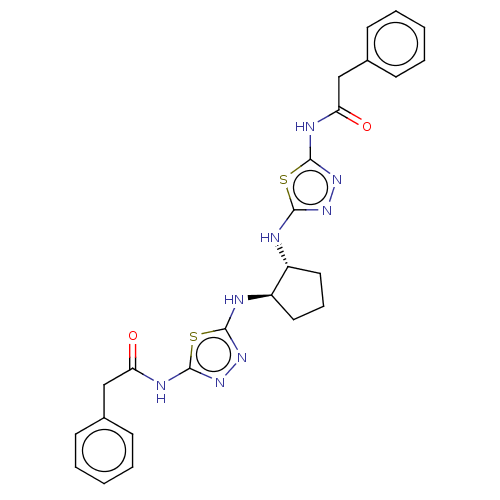
(CHEMBL4437956)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCC[C@H]2Nc2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(14-16-8-3-1-4-9-16)28-24-32-30-22(36-24)26-18-12-7-13-19(18)27-23-31-33-25(37-23)29-21(35)15-17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-15H2,(H,26,30)(H,27,31)(H,28,32,34)(H,29,33,35)/t18-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468002

(CHEMBL4291436)Show SMILES CNc1nc2ccccc2n1-c1nc(cc(n1)C1(CC1)S(C)(=O)=O)N1CCOC[C@H]1C |r| Show InChI InChI=1S/C21H26N6O3S/c1-14-13-30-11-10-26(14)18-12-17(21(8-9-21)31(3,28)29)24-20(25-18)27-16-7-5-4-6-15(16)23-19(27)22-2/h4-7,12,14H,8-11,13H2,1-3H3,(H,22,23)/t14-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HT29 cells after 60 mins by Hoechst 33258 staining-based assay |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468006

(CHEMBL4294006)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-n1c(N)nc2ccc(F)cc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23FN6O3S/c1-12-11-30-8-7-26(12)17-10-16(20(5-6-20)31(2,28)29)24-19(25-17)27-15-9-13(21)3-4-14(15)23-18(27)22/h3-4,9-10,12H,5-8,11H2,1-2H3,(H2,22,23)/t12-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427321
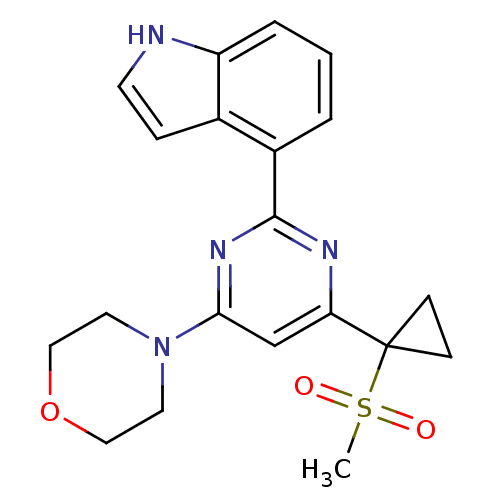
(CHEMBL2325704)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H22N4O3S/c1-28(25,26)20(6-7-20)17-13-18(24-9-11-27-12-10-24)23-19(22-17)15-3-2-4-16-14(15)5-8-21-16/h2-5,8,13,21H,6-7,9-12H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM103467

(US8552004, 2.03)Show SMILES CNc1nc2ccccc2n1-c1nc(cc(n1)C1(CC1)[S@](C)(=N)=O)N1CCOC[C@H]1C |r| Show InChI InChI=1S/C21H27N7O2S/c1-14-13-30-11-10-27(14)18-12-17(21(8-9-21)31(3,22)29)25-20(26-18)28-16-7-5-4-6-15(16)24-19(28)23-2/h4-7,12,14H,3,8-11,13H2,1-2H3,(H2,22,29)(H,23,24)/t14-,31?/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HT29 cells after 60 mins by Hoechst 33258 staining-based assay |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468008

(CHEMBL4280793)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-n1c(N)nc2c(F)cccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23FN6O3S/c1-12-11-30-9-8-26(12)16-10-15(20(6-7-20)31(2,28)29)23-19(24-16)27-14-5-3-4-13(21)17(14)25-18(27)22/h3-5,10,12H,6-9,11H2,1-2H3,(H2,22,25)/t12-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514985

(CHEMBL4573635)Show SMILES C[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7OS/c1-13(14-6-3-2-4-7-14)17(27)22-19-25-24-18(28-19)21-15-9-11-26(12-15)16-8-5-10-20-23-16/h2-8,10,13,15H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t13-,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at progesterone receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468015
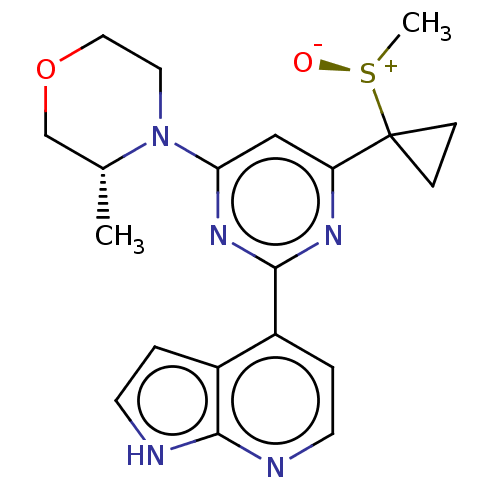
(CHEMBL4284359)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1ccnc2[nH]ccc12)C1(CC1)[S@+](C)[O-] |r| Show InChI InChI=1S/C20H23N5O2S/c1-13-12-27-10-9-25(13)17-11-16(20(5-6-20)28(2)26)23-19(24-17)15-4-8-22-18-14(15)3-7-21-18/h3-4,7-8,11,13H,5-6,9-10,12H2,1-2H3,(H,21,22)/t13-,28+/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50468003

(CHEMBL4281240)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cncc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H23N5O3S/c1-13-12-28-8-7-25(13)18-9-17(20(4-5-20)29(2,26)27)23-19(24-18)15-10-21-11-16-14(15)3-6-22-16/h3,6,9-11,13,22H,4-5,7-8,12H2,1-2H3/t13-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HT29 cells after 60 mins by Hoechst 33258 staining-based assay |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125081
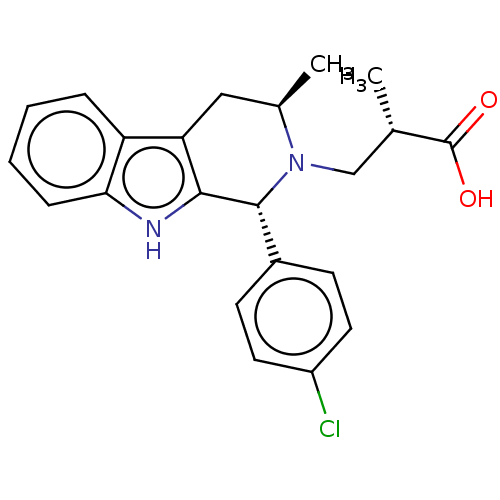
(CHEMBL3622997)Show SMILES C[C@@H](CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C22H23ClN2O2/c1-13(22(26)27)12-25-14(2)11-18-17-5-3-4-6-19(17)24-20(18)21(25)15-7-9-16(23)10-8-15/h3-10,13-14,21,24H,11-12H2,1-2H3,(H,26,27)/t13-,14+,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at progesterone receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514975

(CHEMBL4473143)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NC[C@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(13-17-7-3-1-4-8-17)27-23-30-29-22(36-23)26-15-19-11-12-33(16-19)25-32-31-24(37-25)28-21(35)14-18-9-5-2-6-10-18/h1-10,19H,11-16H2,(H,26,29)(H,27,30,34)(H,28,31,35)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM103471

(BDBM50456371 | US8552004, 2.11)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cncc2[nH]ccc12)C1(CC1)S(C)(=N)=O |r| Show InChI InChI=1S/C20H24N6O2S/c1-13-12-28-8-7-26(13)18-9-17(20(4-5-20)29(2,21)27)24-19(25-18)15-10-22-11-16-14(15)3-6-23-16/h3,6,9-11,13,23H,2,4-5,7-8,12H2,1H3,(H2,21,27)/t13-,29?/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HT29 cells after 60 mins by Hoechst 33258 staining-based assay |
J Med Chem 61: 9889-9907 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01187
BindingDB Entry DOI: 10.7270/Q2S46VPQ |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50150109
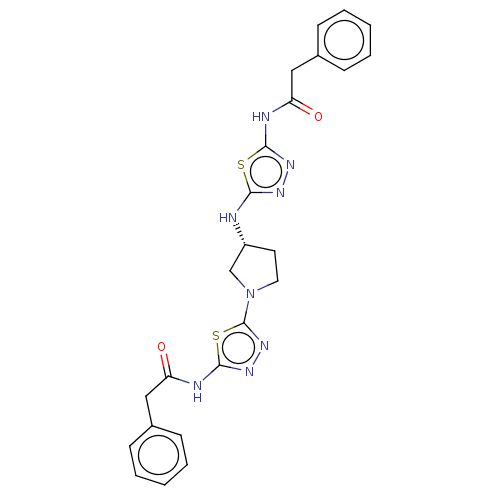
(CHEMBL3770355)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C24H24N8O2S2/c33-19(13-16-7-3-1-4-8-16)26-22-29-28-21(35-22)25-18-11-12-32(15-18)24-31-30-23(36-24)27-20(34)14-17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,25,28)(H,26,29,33)(H,27,30,34)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data