

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537558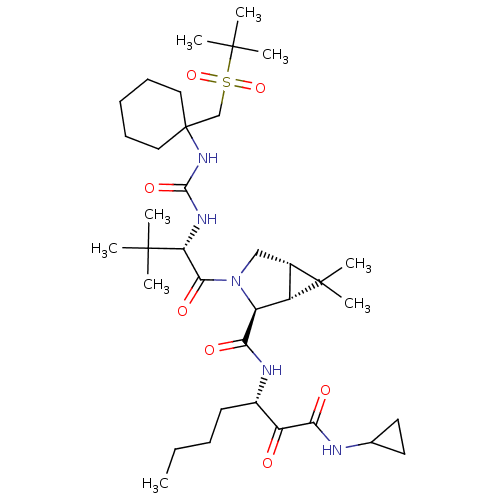 (Narlaprevir | SCH 900518 | SCH-900518) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537563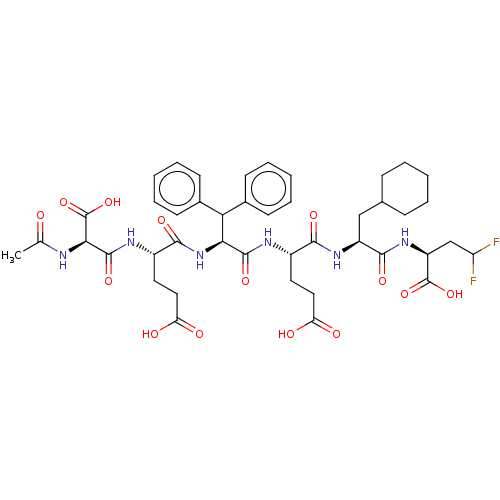 (CHEMBL4634405) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor from human A431 cell membranes | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50103860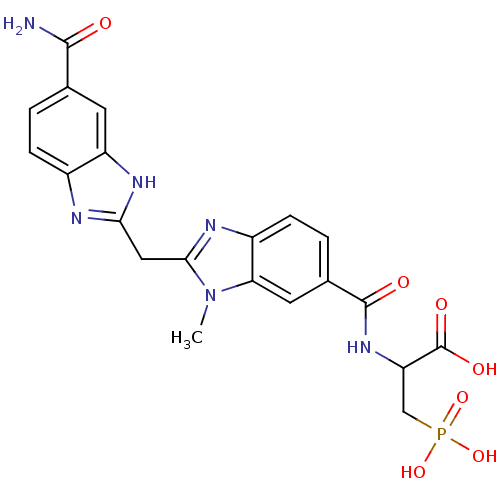 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537564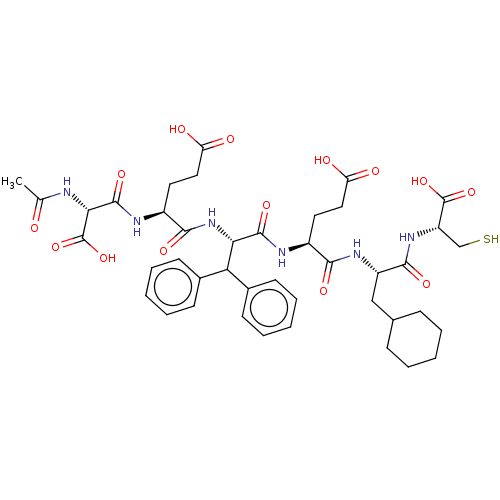 (CHEMBL4644516) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537569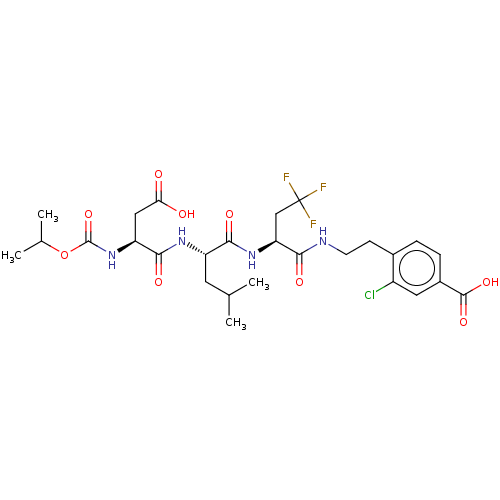 (CHEMBL4646328) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50495950 (Danoprevir | R-05190591 | R05190591 | RO-5190591 |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537589 (CHEMBL4287268) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537581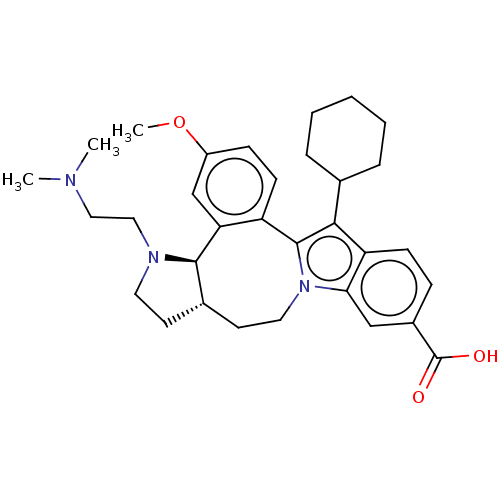 (CHEMBL4638107) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537583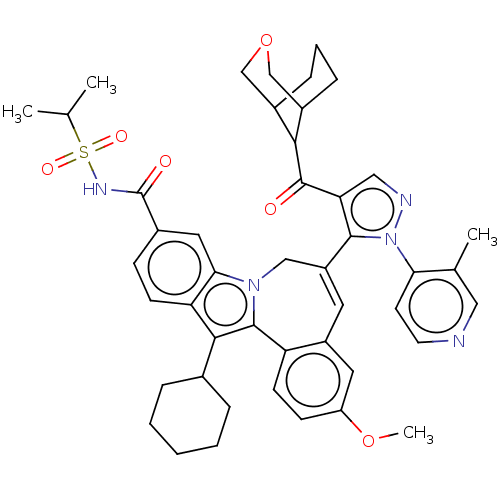 (CHEMBL4638630) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537585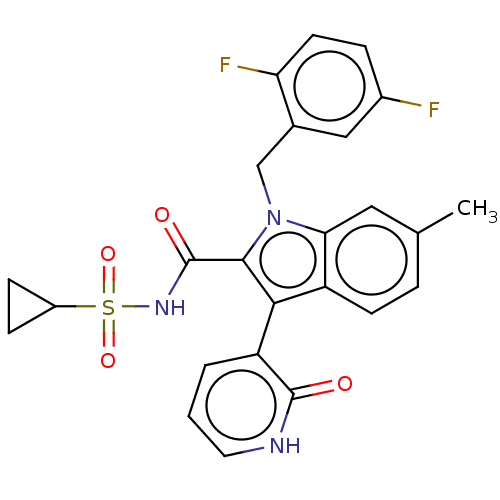 (CHEMBL4646449) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537579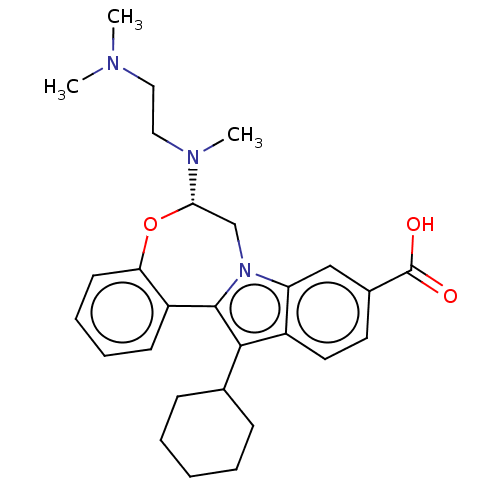 (CHEMBL4635161) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537587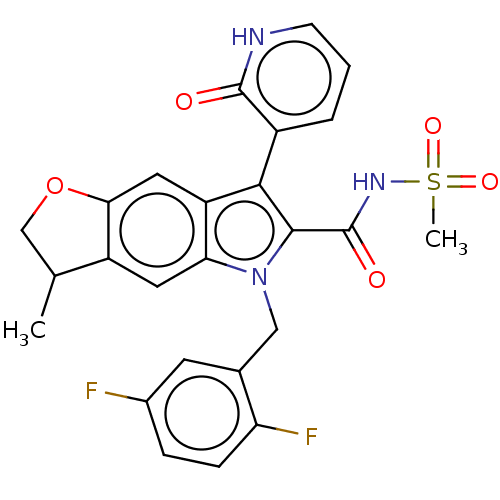 (CHEMBL4633142) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537578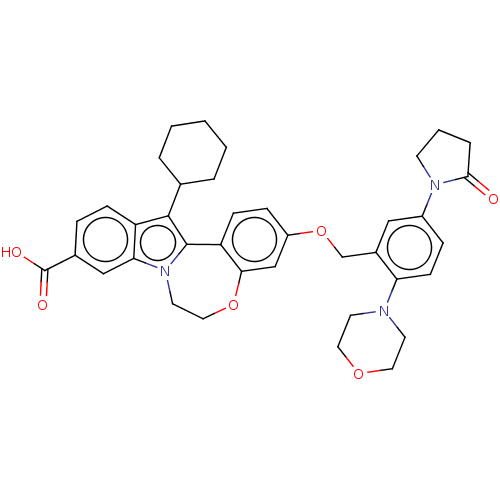 (CHEMBL245328) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50379197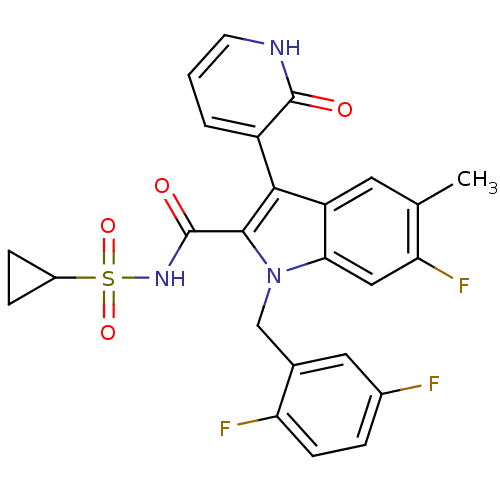 (CHEMBL2011266) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537574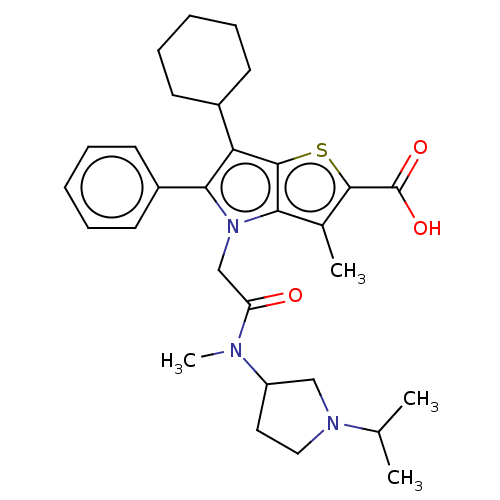 (CHEMBL4643906) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537575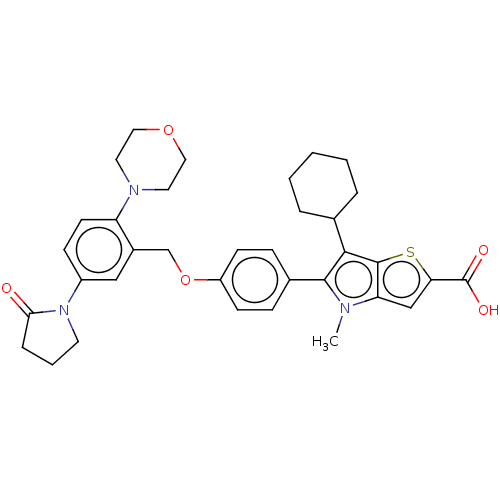 (CHEMBL4638877) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537588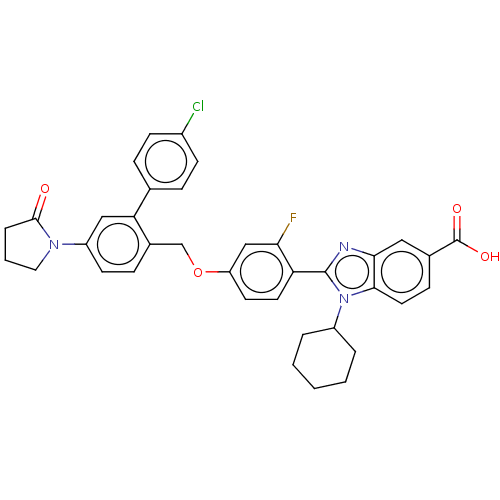 (CHEMBL4643189) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142061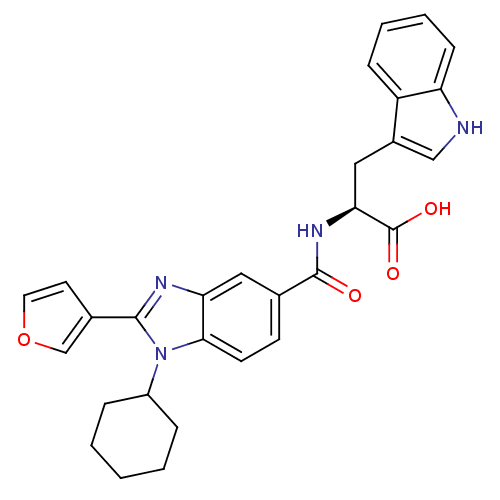 ((2S)-2-(1-cyclohexyl-2-(furan-3-yl)-1H-benzo[d]imi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50081635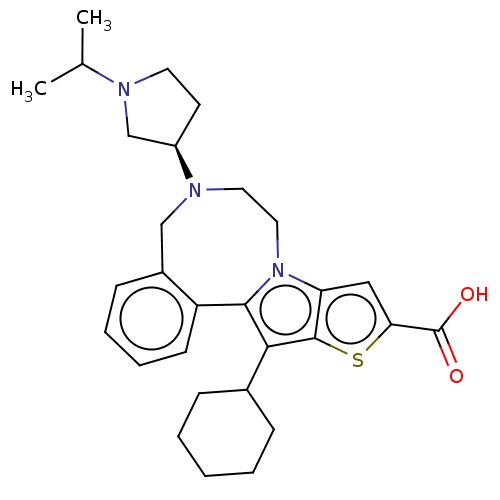 (CHEMBL3422107) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537580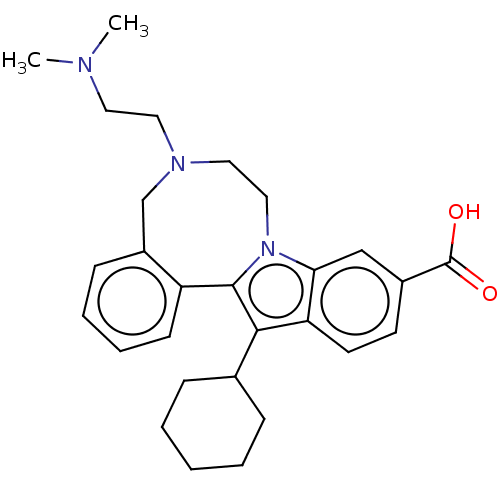 (CHEMBL480816) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537566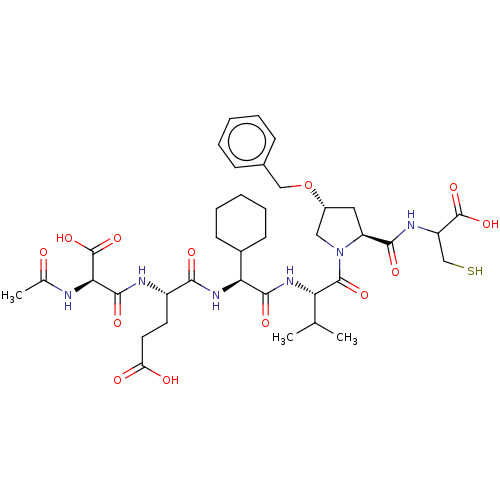 (CHEMBL4640558) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537586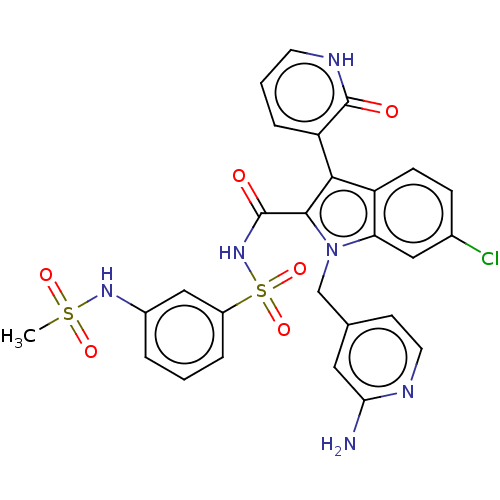 (CHEMBL4641917) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537571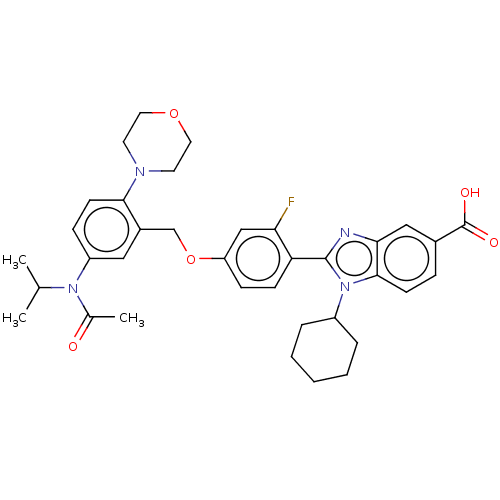 (CHEMBL390883) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537565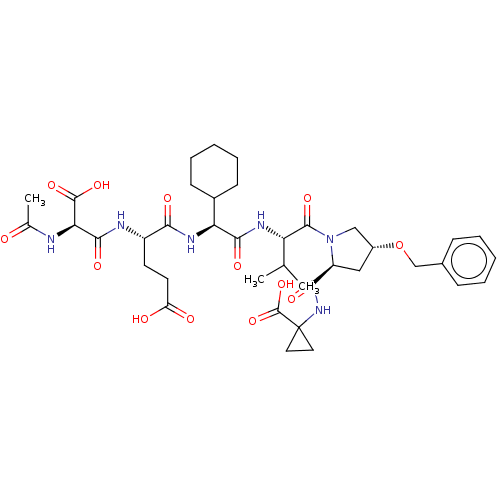 (CHEMBL4649647) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537584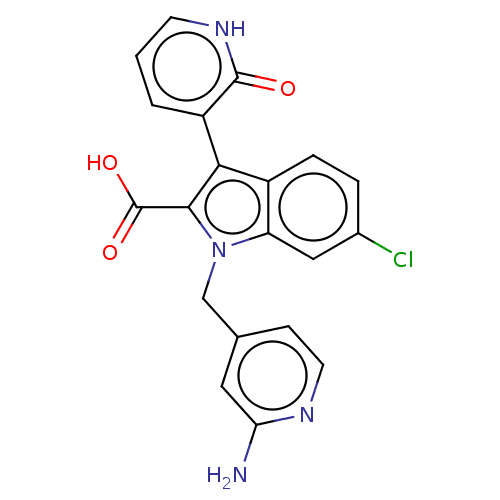 (CHEMBL4636786) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50187149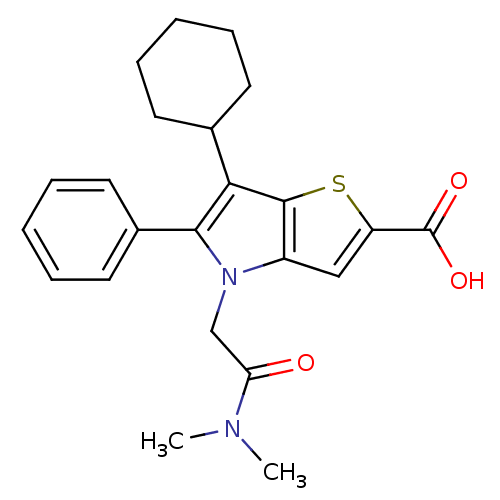 (6-cyclohexyl-4-(2-(dimethylamino)-2-oxoethyl)-5-ph...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537582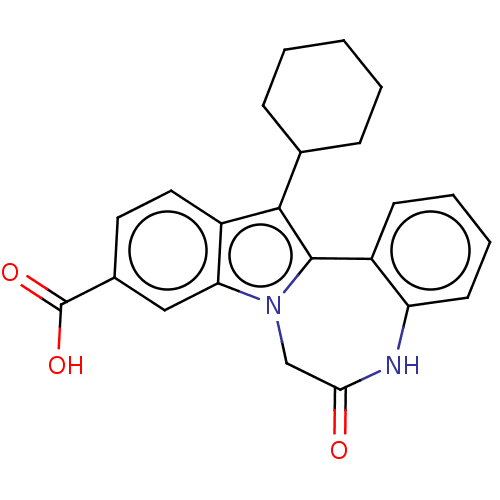 (CHEMBL1783101) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50137504 (1-Cyclohexyl-2-furan-2-yl-1H-benzoimidazole-5-carb...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537572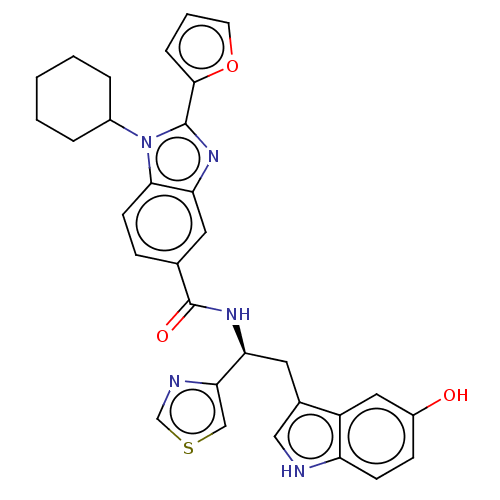 (CHEMBL1087567) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50370763 (CHEMBL1203926) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537576 (CHEMBL4633554) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50160199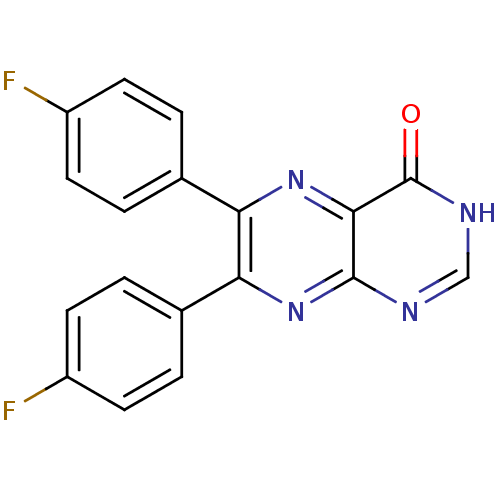 (6,7-Bis-(4-fluoro-phenyl)-pteridin-4-ol | CHEMBL18...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537573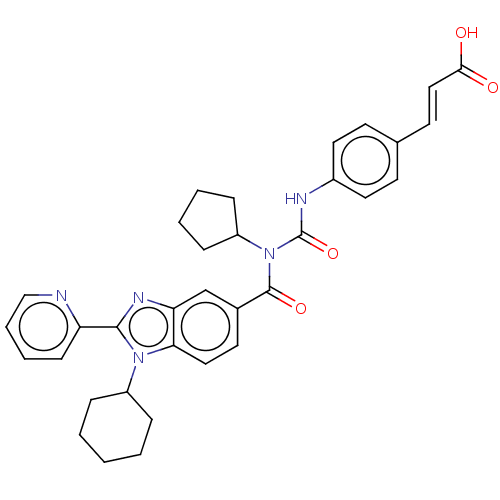 (CHEMBL4642429) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50537577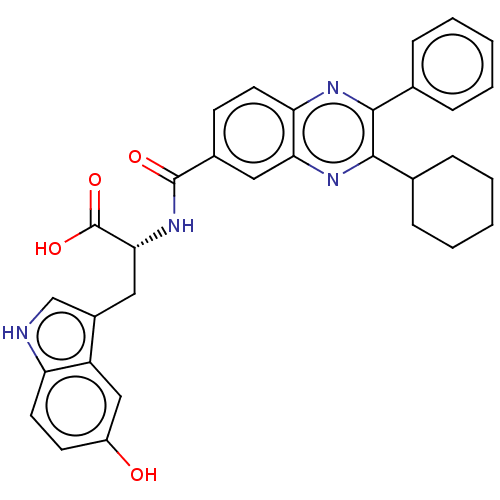 (CHEMBL4639473) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537567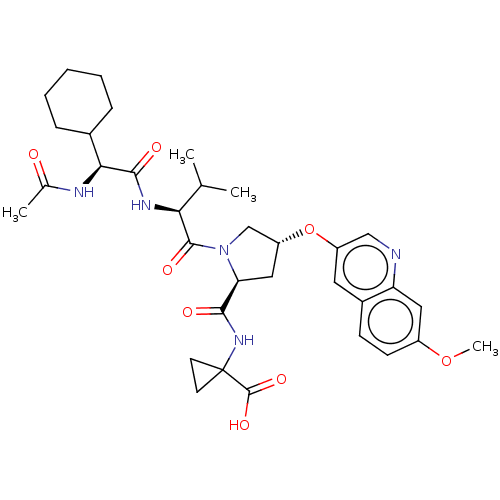 (CHEMBL4632418) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50137488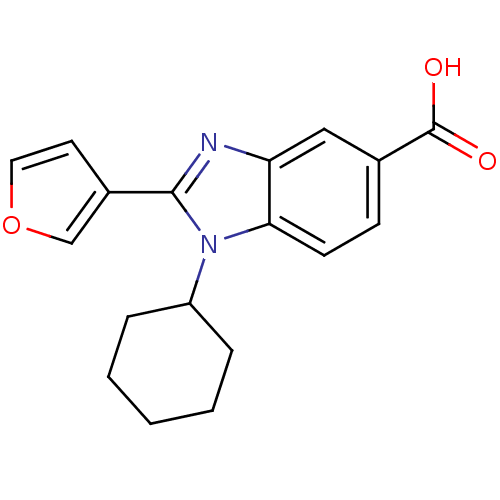 (1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazole-5-carb...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537559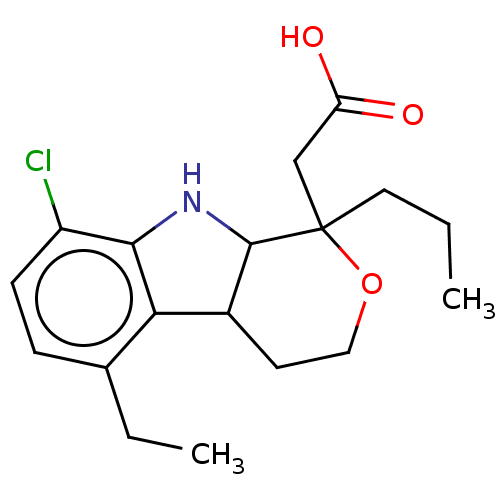 (CHEMBL4640738) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50181949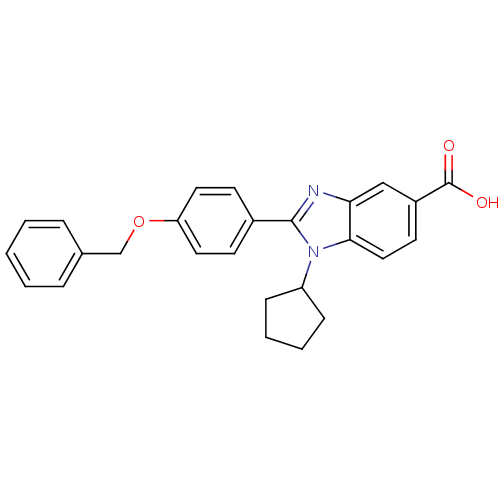 (2-(4-(benzyloxy)phenyl)-1-cyclopentyl-1H-benzo[d]i...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537591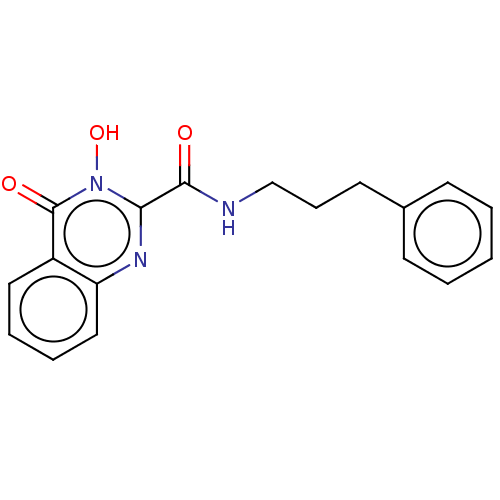 (CHEMBL3617579) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50537568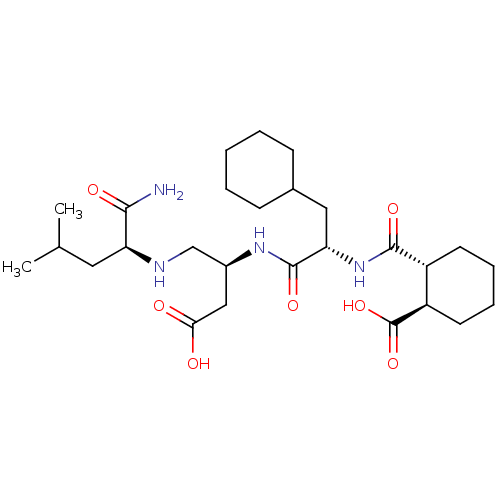 (CHEMBL4641913) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537590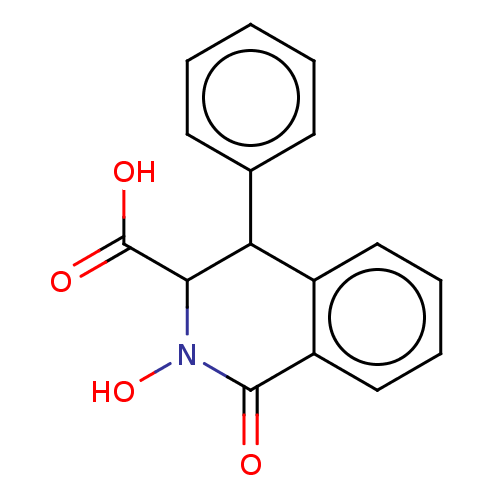 (CHEMBL4641216) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537570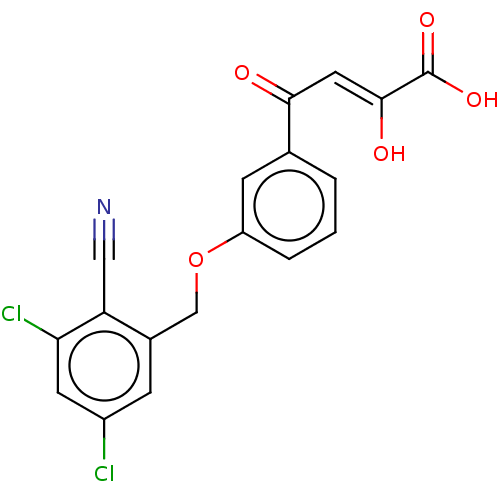 (CHEMBL474496) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537561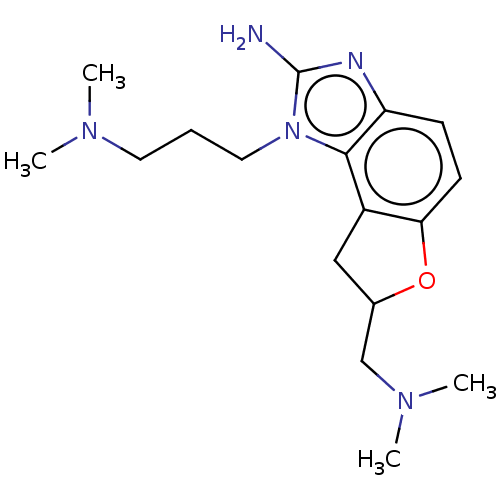 (CHEMBL388502) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537560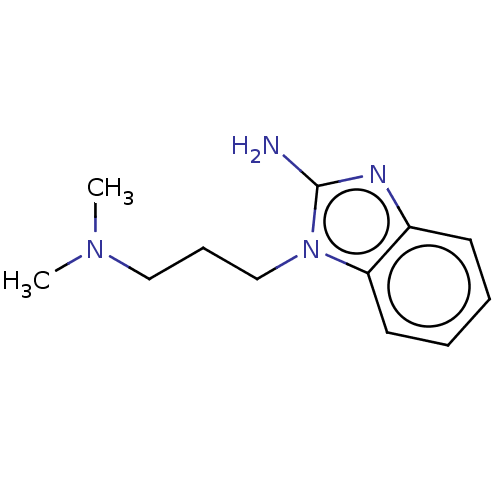 (CHEMBL427101) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50537562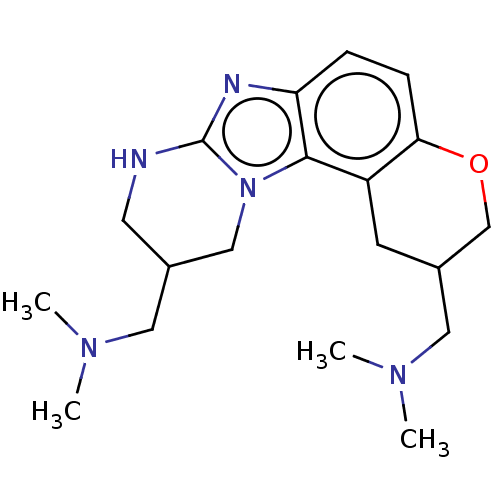 (CHEMBL223720) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1016/j.ejmech.2018.12.045 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||