

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492288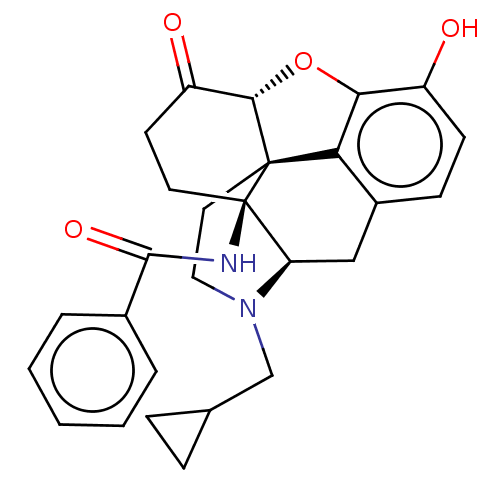 (CHEMBL2397015) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266857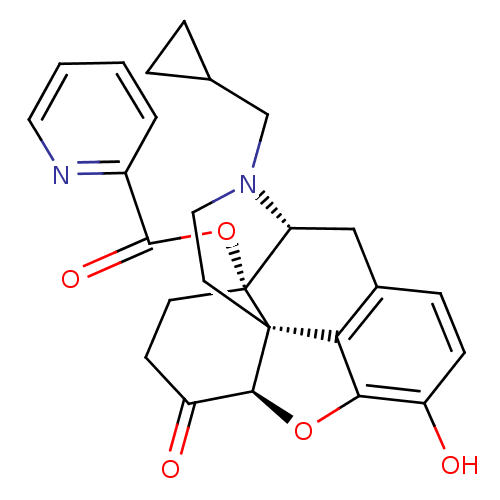 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494375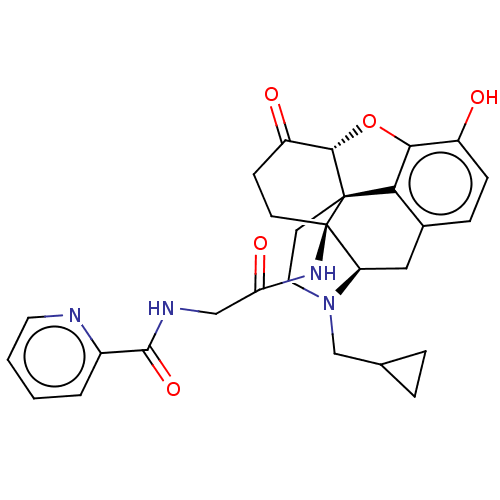 (CHEMBL3086756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492292 (CHEMBL2397021) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494377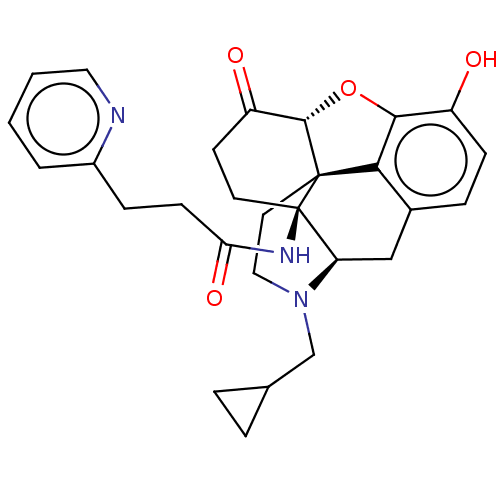 (CHEMBL3086755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50494376 (CHEMBL3086754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492287 (CHEMBL2397017) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017698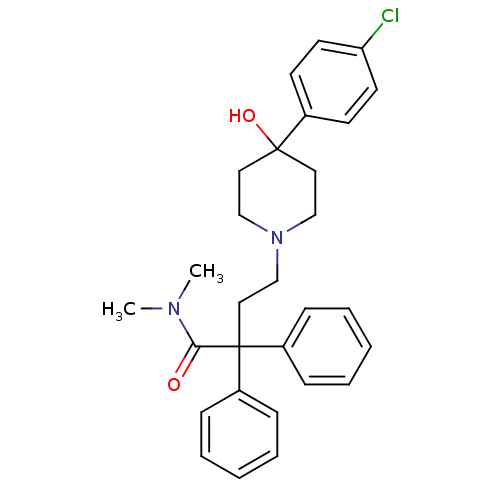 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492291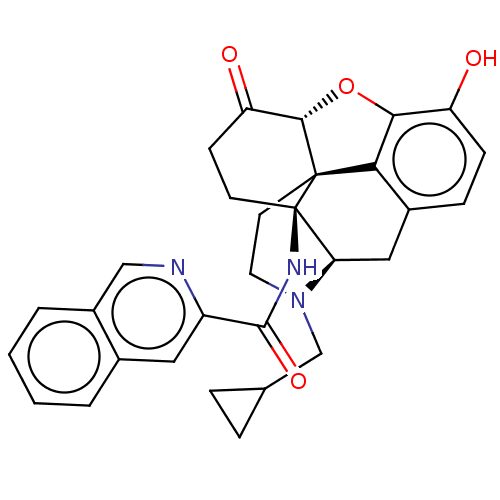 (CHEMBL2397016) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494376 (CHEMBL3086754) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494375 (CHEMBL3086756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494377 (CHEMBL3086755) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492286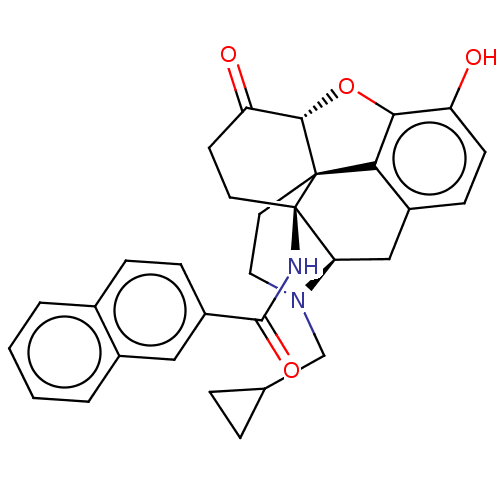 (CHEMBL2397019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492290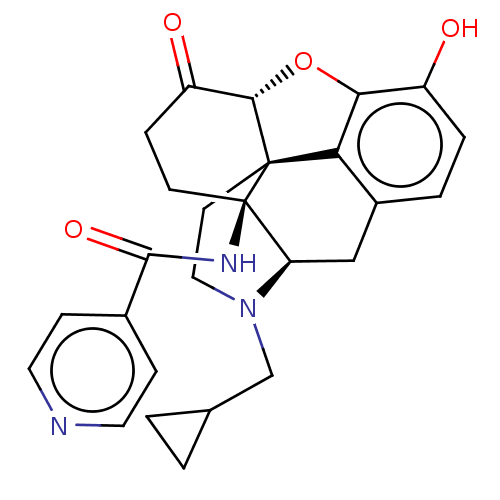 (CHEMBL2397022) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50492289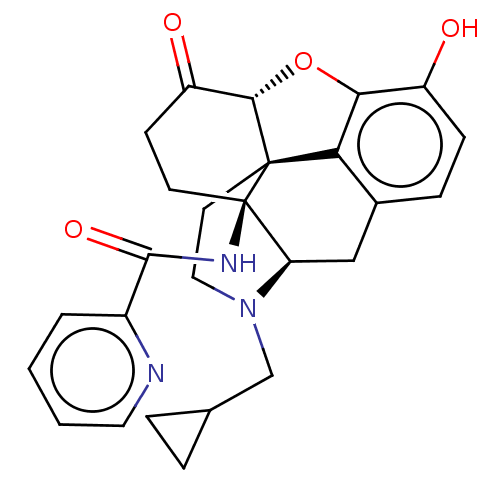 (CHEMBL2397020) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50494375 (CHEMBL3086756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NTI from delta opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585129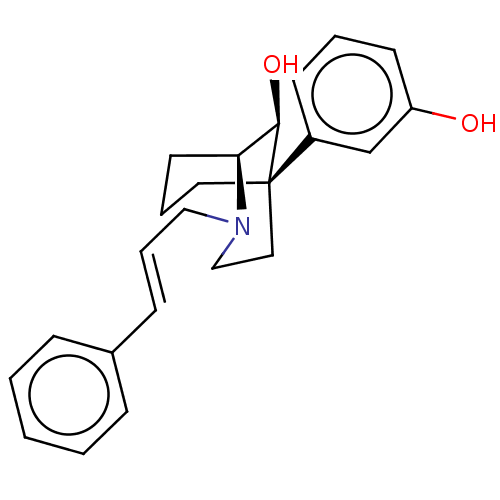 (CHEMBL5077645) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066282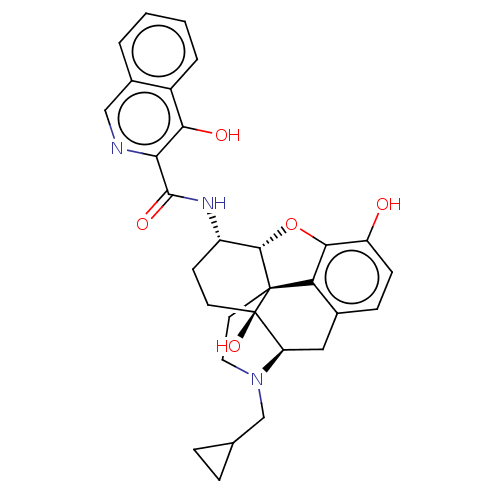 (CHEMBL2417568) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066279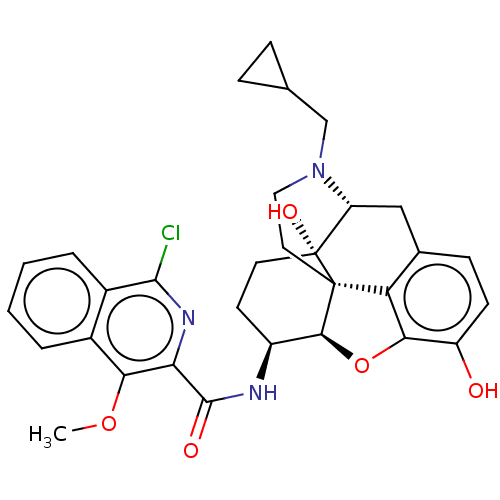 (CHEMBL2419121) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50066278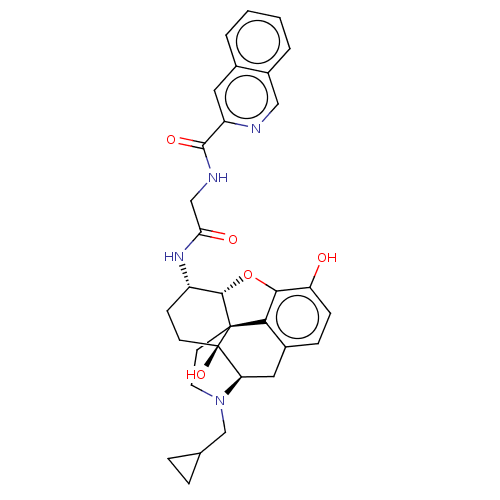 (CHEMBL2419120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mouse KOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585128 (CHEMBL5080654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.633 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066277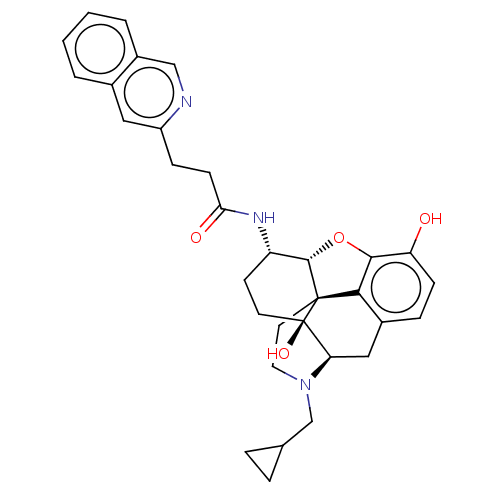 (CHEMBL2419119) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066280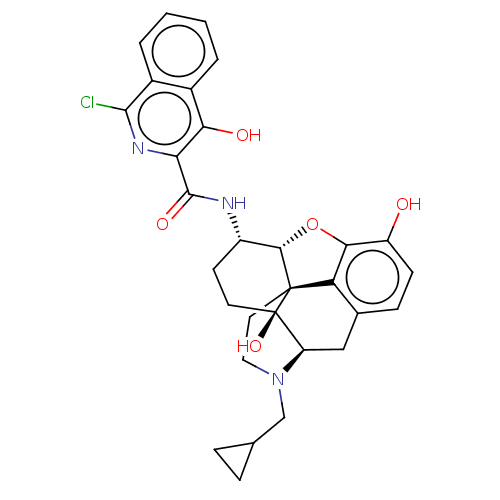 (CHEMBL2419122) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492292 (CHEMBL2397021) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492290 (CHEMBL2397022) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585107 (CHEMBL5085974) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585112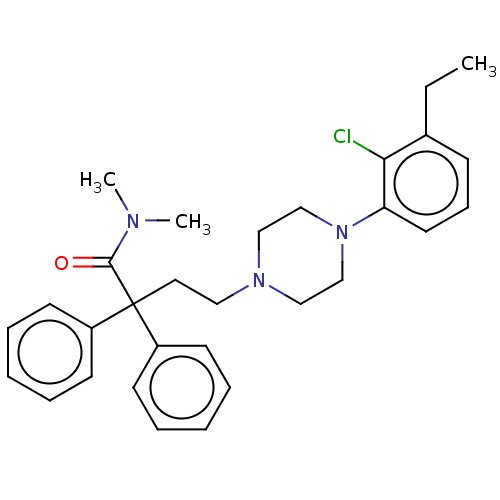 (CHEMBL5088070) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066344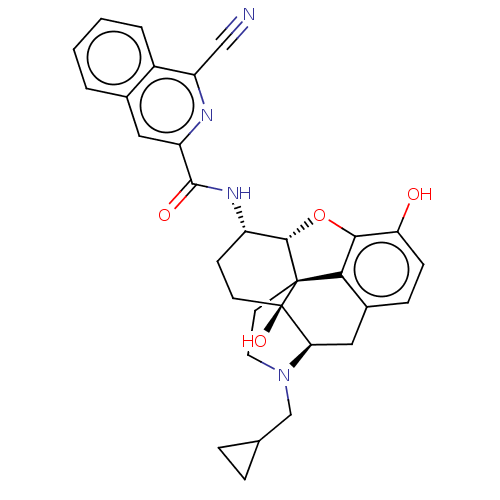 (CHEMBL2419116) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066284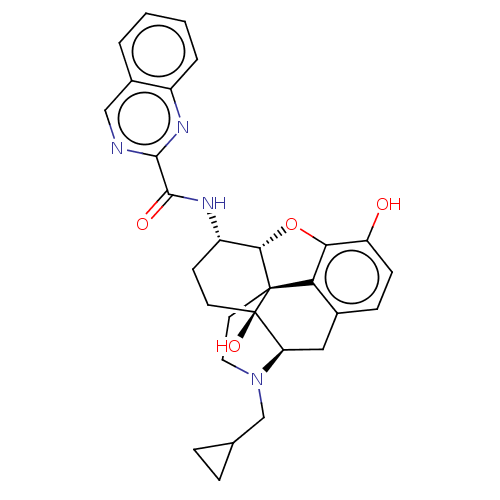 (CHEMBL2419123) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50066276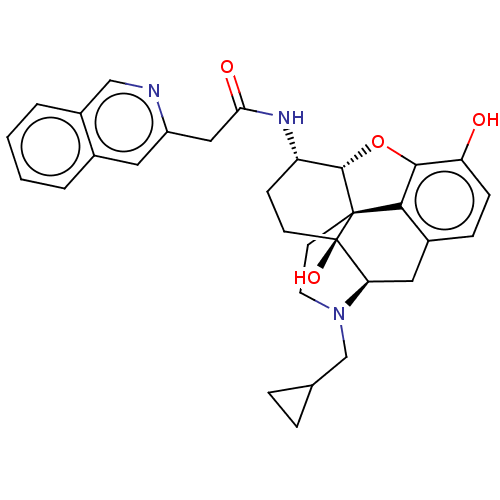 (CHEMBL2419118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mouse KOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50292919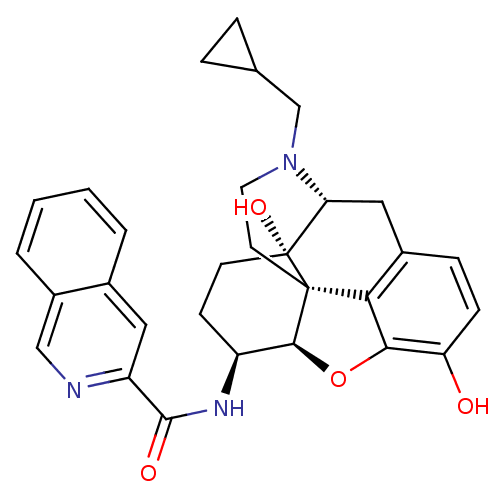 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292920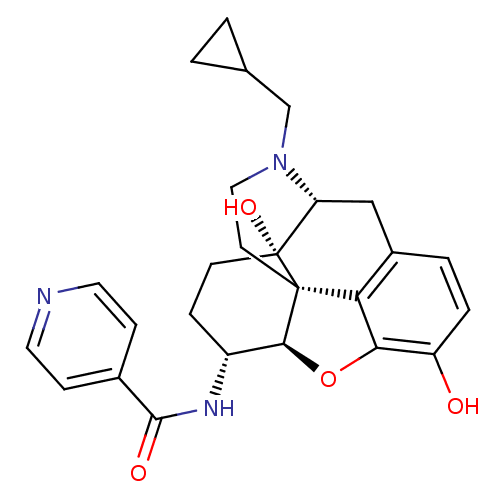 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066276 (CHEMBL2419118) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066285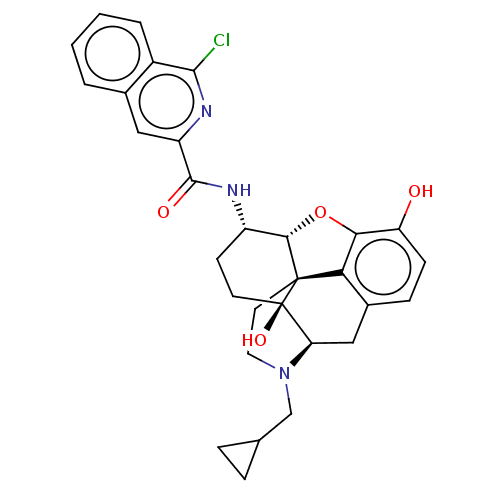 (CHEMBL2419124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D4 receptor expressed in HEK293 cell membranes incubated for 60 mins by microbeta scintill... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266887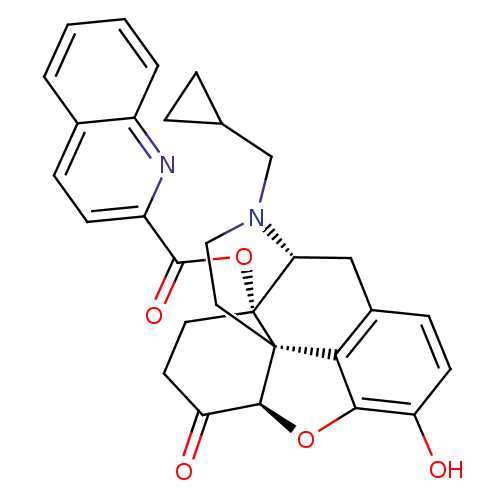 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mouse KOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50492293 (CHEMBL2397018) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NTI from delta opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50492289 (CHEMBL2397020) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50266858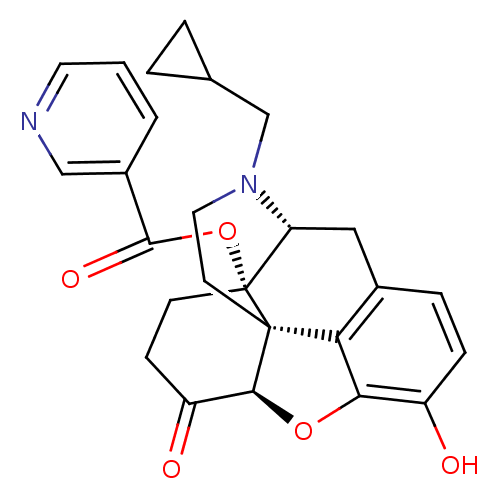 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10-hydroxy-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]NLX from mu opioid receptor (unknown origin) expressed in CHO cell membranes after 1.5 hrs | J Med Chem 56: 9156-69 (2013) Article DOI: 10.1021/jm4012214 BindingDB Entry DOI: 10.7270/Q2JH3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50066277 (CHEMBL2419119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mouse KOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50066359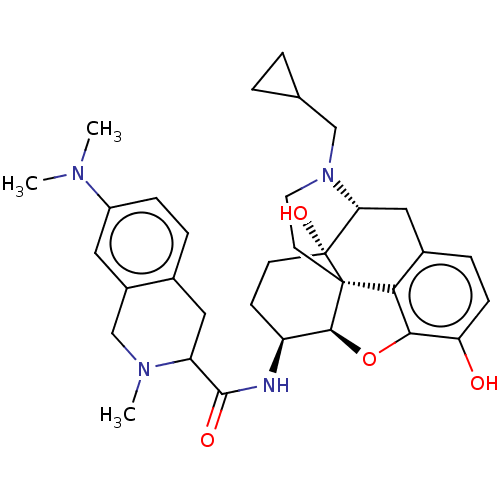 (CHEMBL3401558) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]naloxane from mouse MOR expressed in CHO cells after 1.5 hrs by [35S]GTPgammaS binding assay | Bioorg Med Chem 23: 1701-15 (2015) Article DOI: 10.1016/j.bmc.2015.02.055 BindingDB Entry DOI: 10.7270/Q2PK0HTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50515233 (CHEMBL4551160) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of acetylcholinesterase in Rattus norvegicus (rat) cortex by Ellman method | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50412543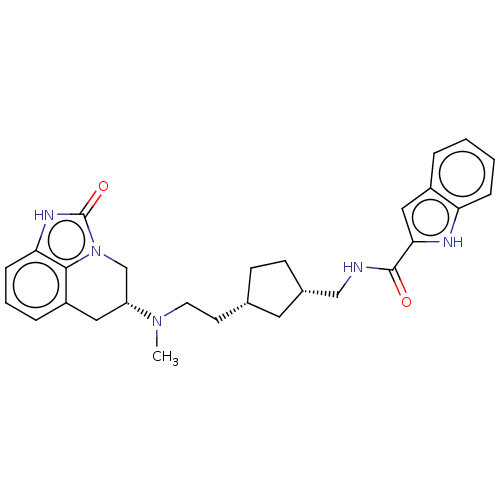 (CHEMBL5273118) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of acetylcholinesterase in Rattus norvegicus (rat) cortex by Ellman method | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 568 total ) | Next | Last >> |