 Found 75 hits with Last Name = 'zhang' and Initial = 'yx'
Found 75 hits with Last Name = 'zhang' and Initial = 'yx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50388686
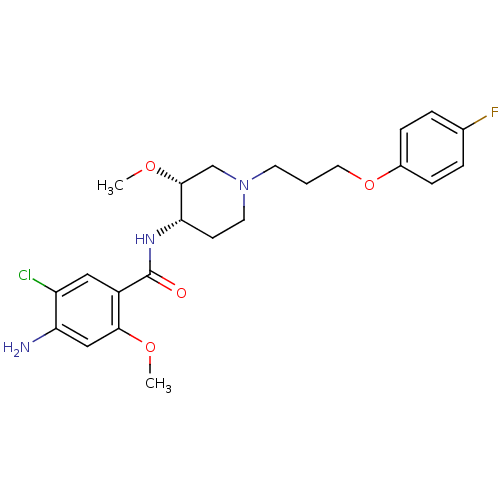
(CHEMBL74656)Show SMILES CO[C@@H]1CN(CCCOc2ccc(F)cc2)CC[C@@H]1NC(=O)c1cc(Cl)c(N)cc1OC |r| Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50103642

(4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...)Show SMILES Cc1cc(cc(C)c1Oc1nc(Nc2ccc(cc2)C#N)nc(N)c1Br)C#N Show InChI InChI=1S/C20H15BrN6O/c1-11-7-14(10-23)8-12(2)17(11)28-19-16(21)18(24)26-20(27-19)25-15-5-3-13(9-22)4-6-15/h3-8H,1-2H3,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16034
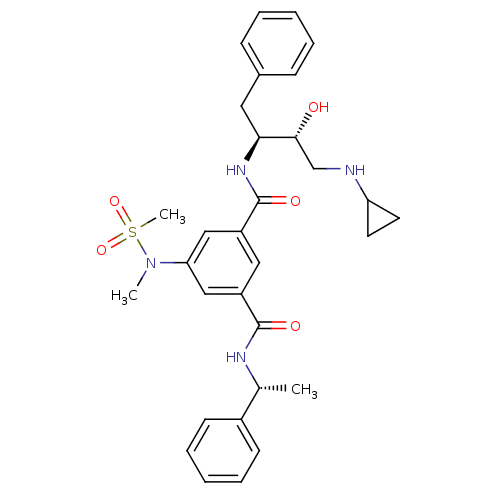
(1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C31H38N4O5S/c1-21(23-12-8-5-9-13-23)33-30(37)24-17-25(19-27(18-24)35(2)41(3,39)40)31(38)34-28(16-22-10-6-4-7-11-22)29(36)20-32-26-14-15-26/h4-13,17-19,21,26,28-29,32,36H,14-16,20H2,1-3H3,(H,33,37)(H,34,38)/t21-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 proteolytic activity |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16034

(1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C31H38N4O5S/c1-21(23-12-8-5-9-13-23)33-30(37)24-17-25(19-27(18-24)35(2)41(3,39)40)31(38)34-28(16-22-10-6-4-7-11-22)29(36)20-32-26-14-15-26/h4-13,17-19,21,26,28-29,32,36H,14-16,20H2,1-3H3,(H,33,37)(H,34,38)/t21-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16034

(1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C31H38N4O5S/c1-21(23-12-8-5-9-13-23)33-30(37)24-17-25(19-27(18-24)35(2)41(3,39)40)31(38)34-28(16-22-10-6-4-7-11-22)29(36)20-32-26-14-15-26/h4-13,17-19,21,26,28-29,32,36H,14-16,20H2,1-3H3,(H,33,37)(H,34,38)/t21-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 using Eu-CEVNLDAEFK-Qsy7 as substrate by HTRF assay |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50582100
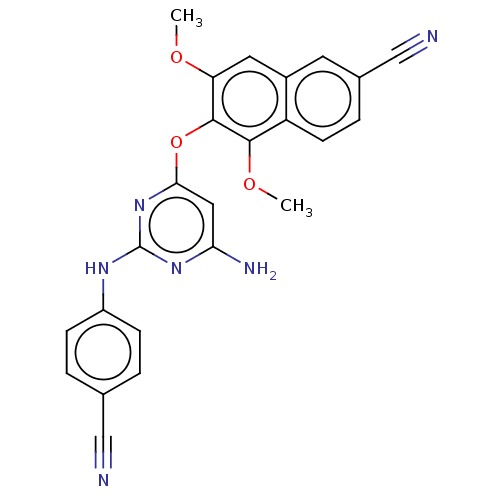
(CHEMBL5073811)Show SMILES COc1cc2cc(ccc2c(OC)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597066
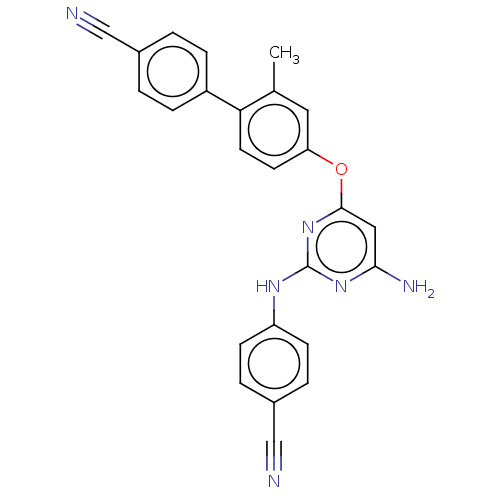
(CHEMBL5202557)Show SMILES Cc1cc(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)ccc1-c1ccc(cc1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16034

(1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C31H38N4O5S/c1-21(23-12-8-5-9-13-23)33-30(37)24-17-25(19-27(18-24)35(2)41(3,39)40)31(38)34-28(16-22-10-6-4-7-11-22)29(36)20-32-26-14-15-26/h4-13,17-19,21,26,28-29,32,36H,14-16,20H2,1-3H3,(H,33,37)(H,34,38)/t21-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in HEK293T cells co-transfected APP with NFEV mutation at proteolytic site by sAPP_NF cell based assay |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597061
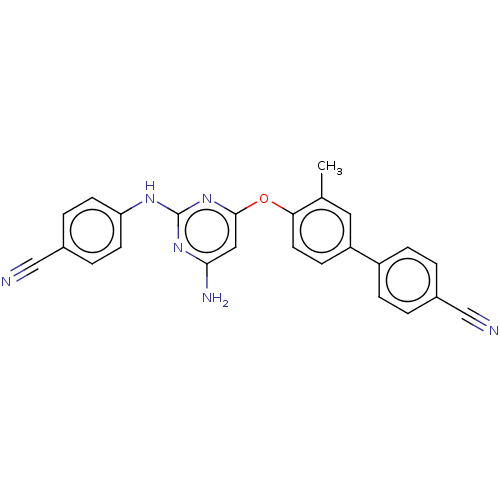
(CHEMBL5176960)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(cc1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597065
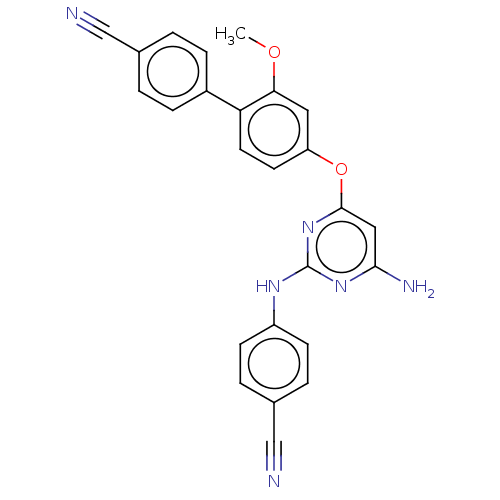
(CHEMBL5196804)Show SMILES COc1cc(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)ccc1-c1ccc(cc1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597064
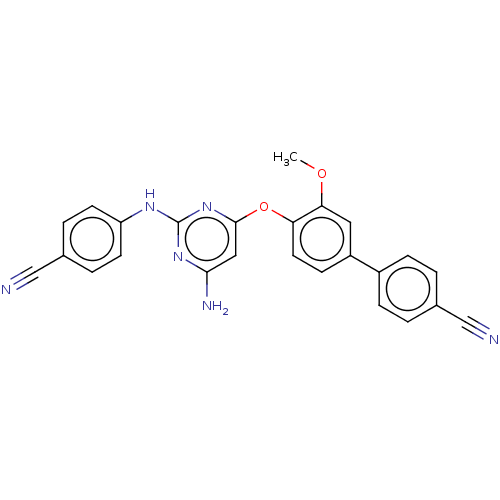
(CHEMBL5200580)Show SMILES COc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(cc1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597052
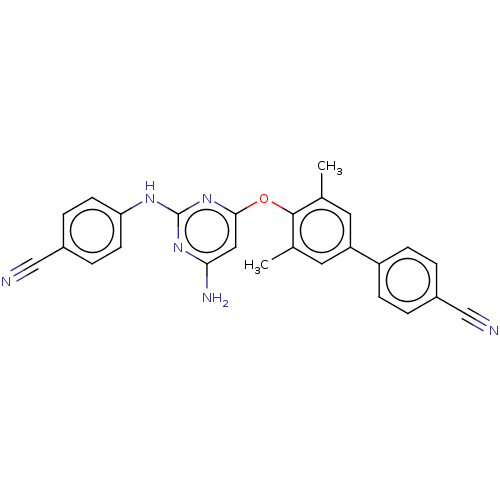
(CHEMBL5195539)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(cc1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597062

(CHEMBL5204633)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(C#N)c(F)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597055
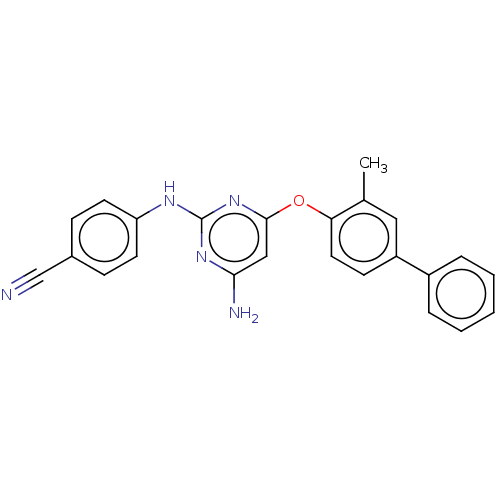
(CHEMBL5199364)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597050
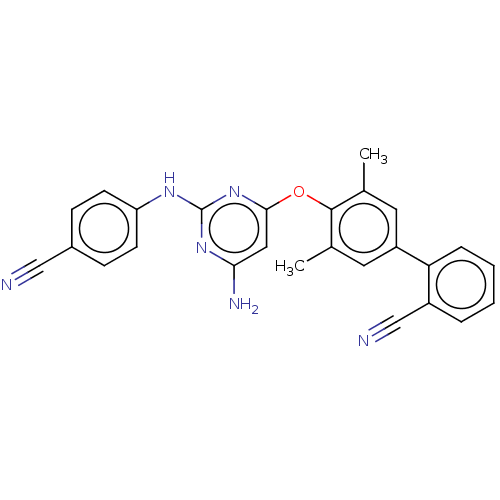
(CHEMBL5175527)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597054
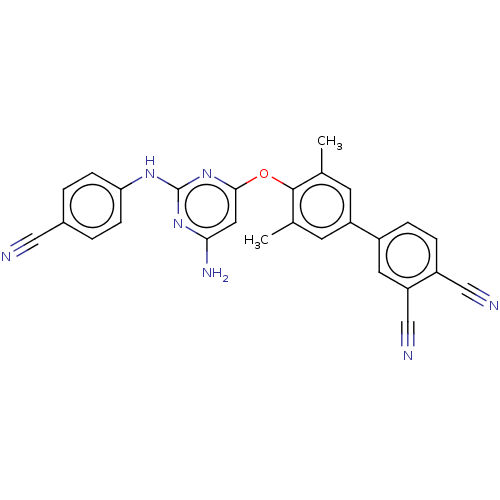
(CHEMBL5202111)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(C#N)c(c1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597060
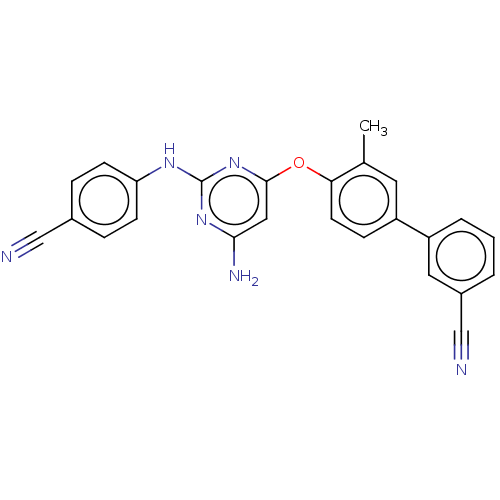
(CHEMBL5183840)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1cccc(c1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597051

(CHEMBL5185715)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1cccc(c1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597056
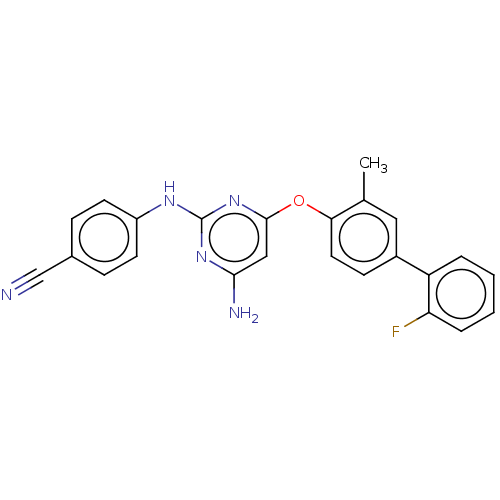
(CHEMBL5173442)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1F | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16034

(1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CC1)N(C)S(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C31H38N4O5S/c1-21(23-12-8-5-9-13-23)33-30(37)24-17-25(19-27(18-24)35(2)41(3,39)40)31(38)34-28(16-22-10-6-4-7-11-22)29(36)20-32-26-14-15-26/h4-13,17-19,21,26,28-29,32,36H,14-16,20H2,1-3H3,(H,33,37)(H,34,38)/t21-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in CHO cells co-transfected with human APP with Swedish mutation assessed as amyloid beta1-40 secreti... |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597037

(CHEMBL5191003)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597038

(CHEMBL5184248)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1F | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597053

(CHEMBL5209478)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(C#N)c(F)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597058

(CHEMBL5201454)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(F)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597059
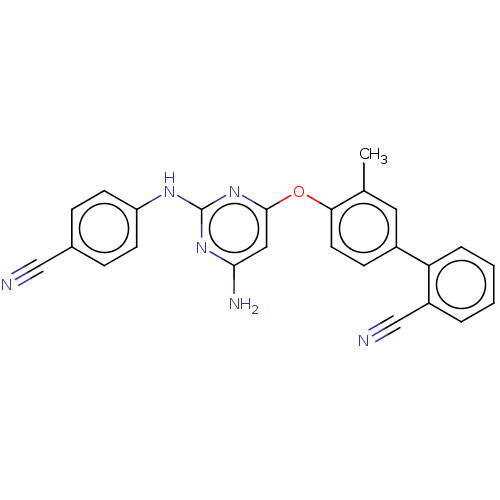
(CHEMBL5197364)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597039

(CHEMBL5194488)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1cccc(F)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597049

(CHEMBL5197260)Show SMILES COc1ccc(cc1)-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597040
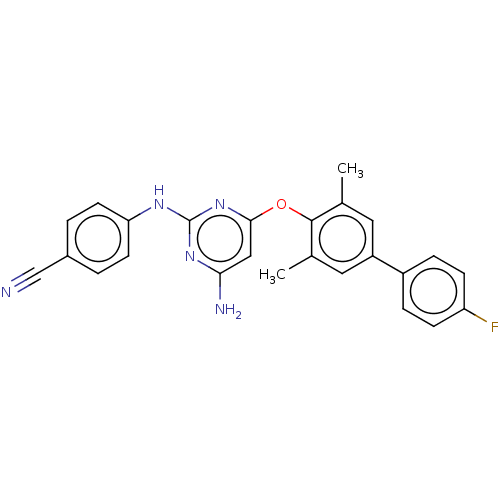
(CHEMBL5203676)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(F)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597046

(CHEMBL5182257)Show SMILES Cc1ccc(cc1)-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597048
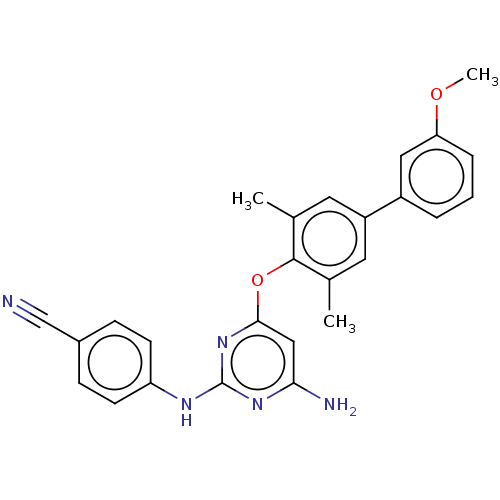
(CHEMBL5192203)Show SMILES COc1cccc(c1)-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597042
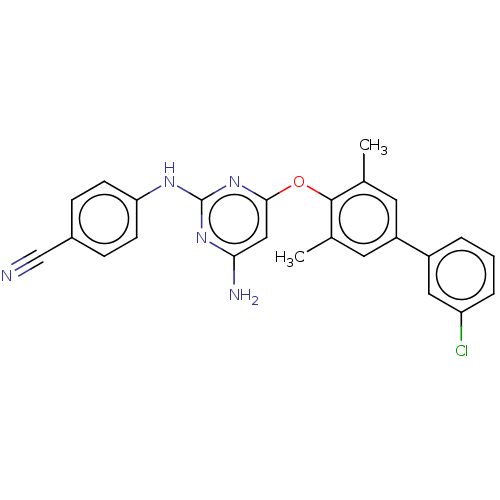
(CHEMBL5175050)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1cccc(Cl)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597063

(CHEMBL5207417)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(C#N)c(c1)C#N | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597041

(CHEMBL5192150)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccccc1Cl | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597057

(CHEMBL5191313)Show SMILES Cc1cc(ccc1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1cccc(F)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597043

(CHEMBL5207844)Show SMILES Cc1cc(cc(C)c1Oc1cc(N)nc(Nc2ccc(cc2)C#N)n1)-c1ccc(Cl)cc1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597044
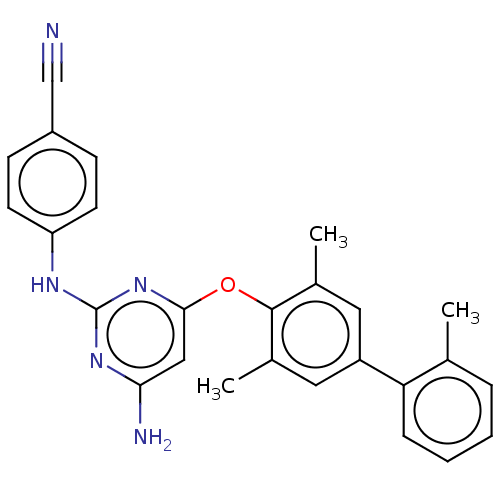
(CHEMBL5196632)Show SMILES Cc1ccccc1-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597047

(CHEMBL5178514)Show SMILES COc1ccccc1-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50597045
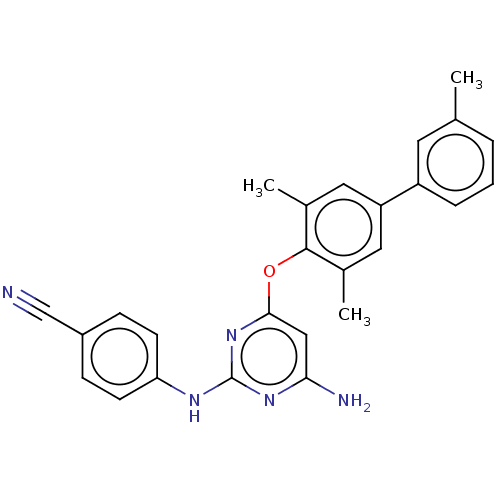
(CHEMBL5180565)Show SMILES Cc1cccc(c1)-c1cc(C)c(Oc2cc(N)nc(Nc3ccc(cc3)C#N)n2)c(C)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50207551
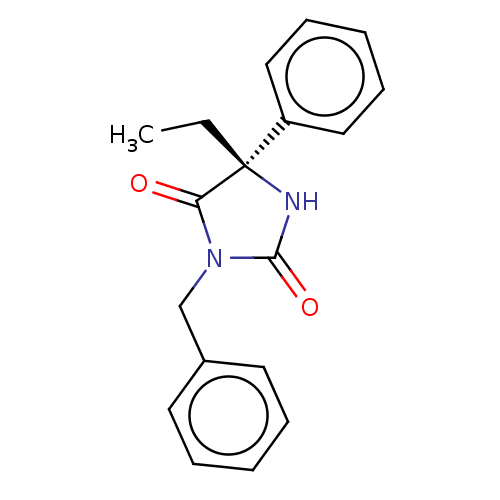
(CHEMBL3977345)Show SMILES CC[C@]1(NC(=O)N(Cc2ccccc2)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C18H18N2O2/c1-2-18(15-11-7-4-8-12-15)16(21)20(17(22)19-18)13-14-9-5-3-6-10-14/h3-12H,2,13H2,1H3,(H,19,22)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
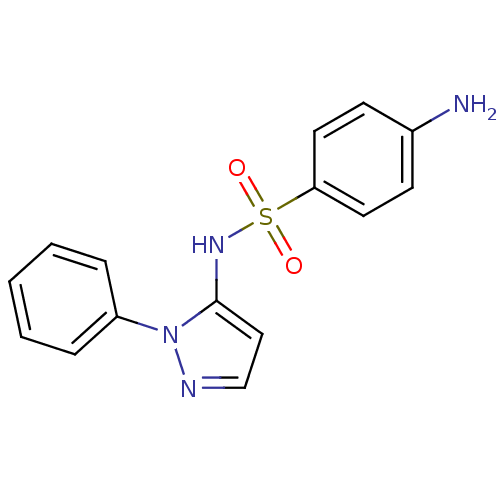
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM222178
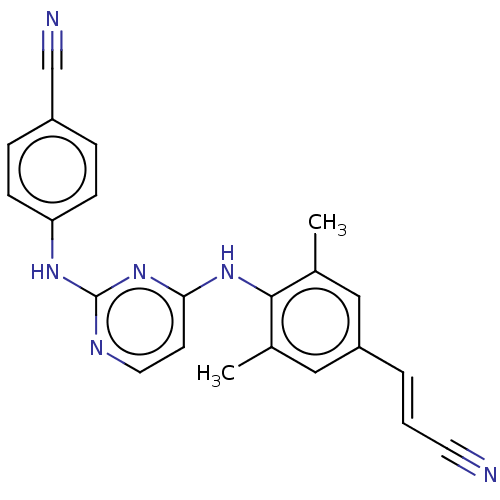
(Rilpivirine)Show SMILES Cc1cc(\C=C\C#N)cc(C)c1Nc1ccnc(Nc2ccc(cc2)C#N)n1 Show InChI InChI=1S/C22H18N6/c1-15-12-18(4-3-10-23)13-16(2)21(15)27-20-9-11-25-22(28-20)26-19-7-5-17(14-24)6-8-19/h3-9,11-13H,1-2H3,(H2,25,26,27,28)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 552 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 757 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 using Eu-CEVNLDAEFK-Qsy7 as substrate by HTRF assay |
Bioorg Med Chem Lett 22: 1408-14 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.034
BindingDB Entry DOI: 10.7270/Q24Q7VGC |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50544150
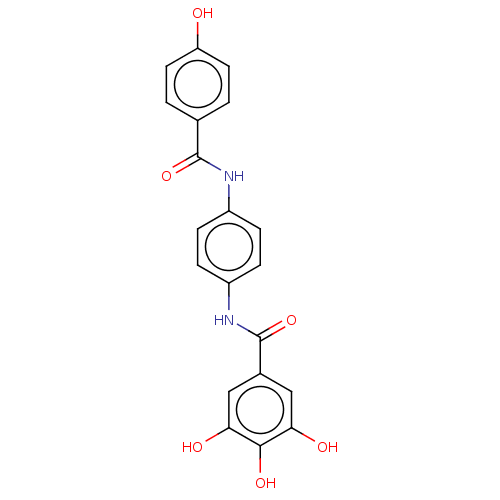
(CHEMBL4641523)Show SMILES Oc1ccc(cc1)C(=O)Nc1ccc(NC(=O)c2cc(O)c(O)c(O)c2)cc1 Show InChI InChI=1S/C20H16N2O6/c23-15-7-1-11(2-8-15)19(27)21-13-3-5-14(6-4-13)22-20(28)12-9-16(24)18(26)17(25)10-12/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-synuclein (unknown origin) aggregation incubated for 3 days by thioflavin T based fluorescence assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115596
BindingDB Entry DOI: 10.7270/Q2ST7TD0 |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50510750
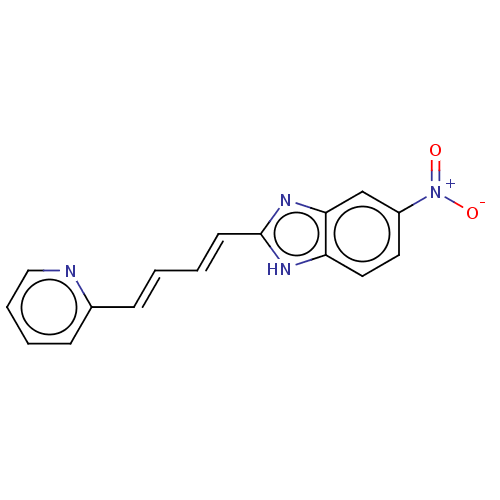
(CHEMBL4551721)Show SMILES [O-][N+](=O)c1ccc2[nH]c(\C=C\C=C\c3ccccn3)nc2c1 Show InChI InChI=1S/C16H12N4O2/c21-20(22)13-8-9-14-15(11-13)19-16(18-14)7-2-1-5-12-6-3-4-10-17-12/h1-11H,(H,18,19)/b5-1+,7-2+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-synuclein aggregation (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 days by thioflavin T fluorescence... |
Bioorg Med Chem 27: 3089-3096 (2019)
Article DOI: 10.1016/j.bmc.2019.05.032
BindingDB Entry DOI: 10.7270/Q290274V |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50544151

(CHEMBL4640362)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(NC(=O)c2cc(O)c(O)c(O)c2)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-12-2-8-16(9-3-12)29(27,28)22-15-6-4-14(5-7-15)21-20(26)13-10-17(23)19(25)18(24)11-13/h2-11,22-25H,1H3,(H,21,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-synuclein (unknown origin) aggregation incubated for 3 days by thioflavin T based fluorescence assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115596
BindingDB Entry DOI: 10.7270/Q2ST7TD0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data