

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome b (Sus scrofa) | BDBM50487138 (CHEMBL2251795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487139 (CHEMBL2251793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487144 (CHEMBL2251794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487137 (CHEBI:78780 | DNDI1724943 | PYRACLOSTROBIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487146 (CHEMBL2251796) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487145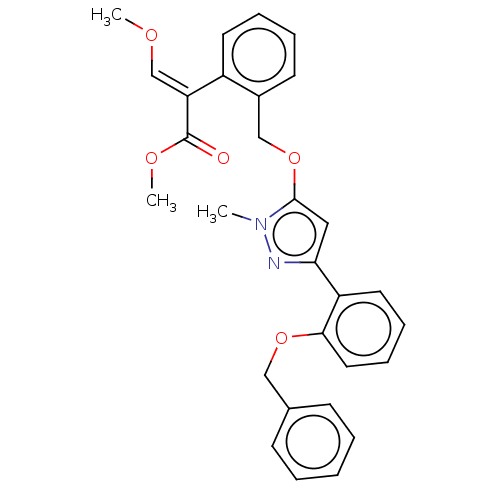 (CHEMBL2251799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487142 (CHEMBL2251798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487143 (CHEMBL2251797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487141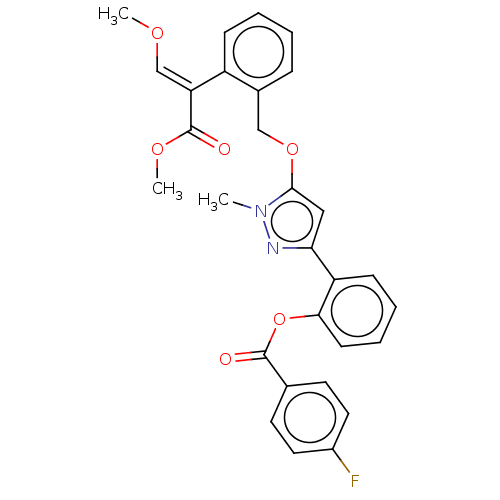 (CHEMBL2251800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487140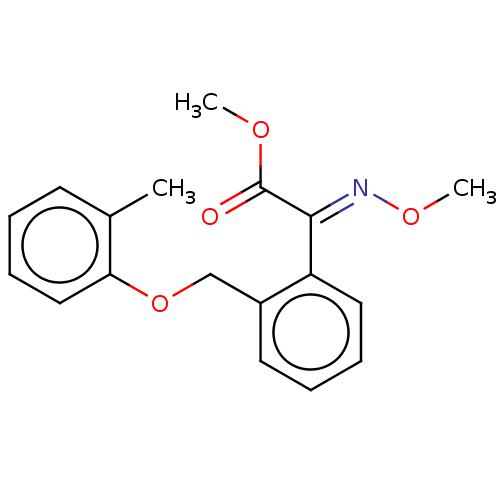 (CHEBI:2962 | Kresoxim-Methyl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487147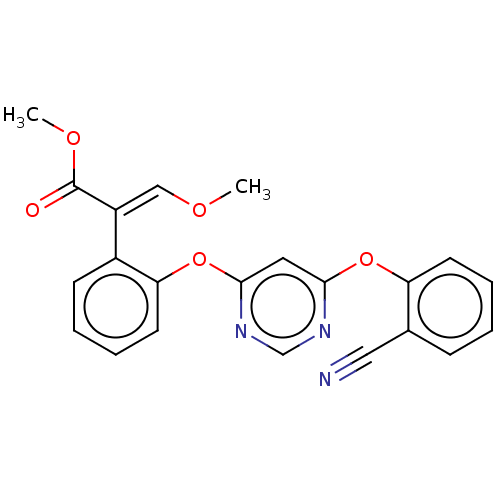 (AZOXYSTROBIN | CHEBI:40909 | DNDI1511705 | TCMDC-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513526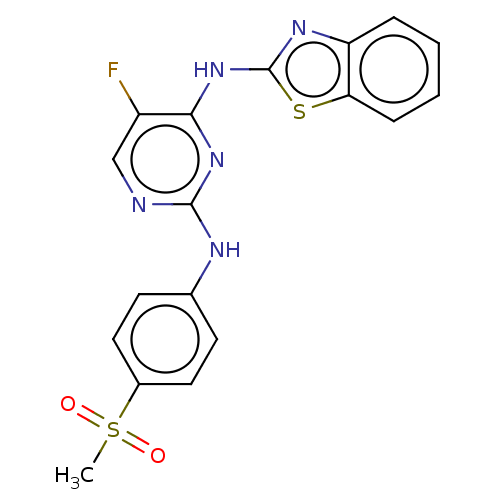 (CHEMBL4526687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513523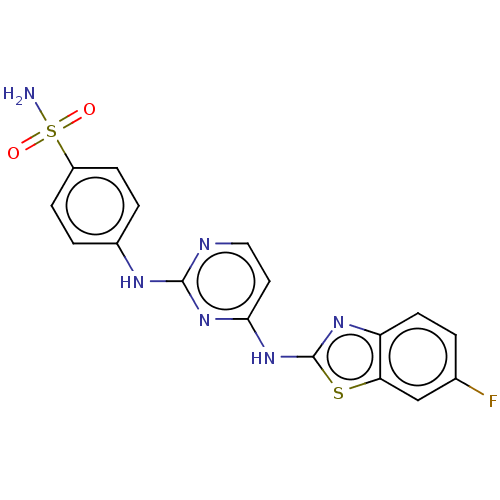 (CHEMBL4454288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513527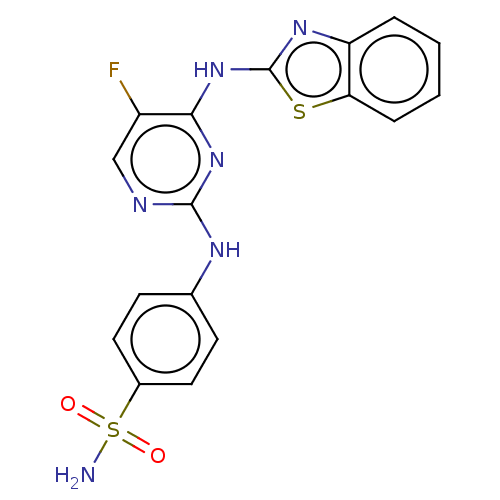 (CHEMBL4435968) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513522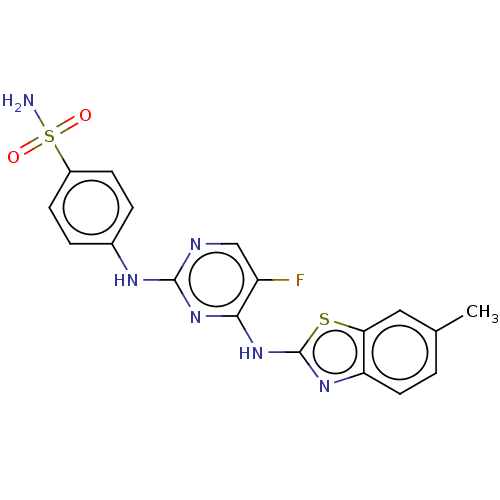 (CHEMBL4528300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513524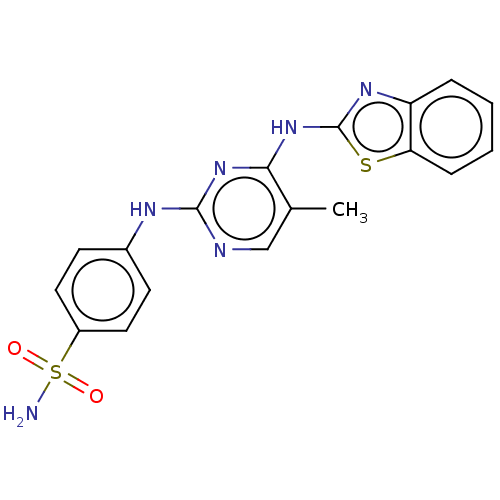 (CHEMBL4475342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50513525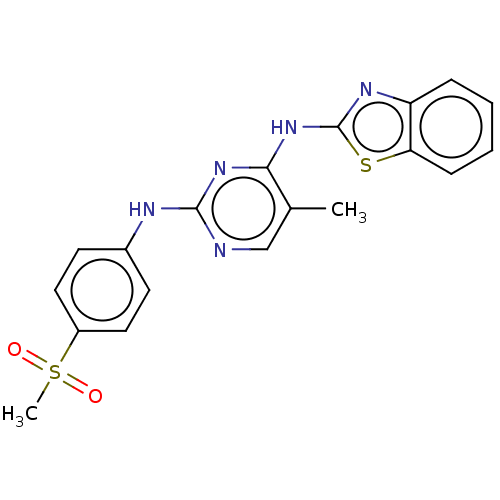 (CHEMBL4543030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50246253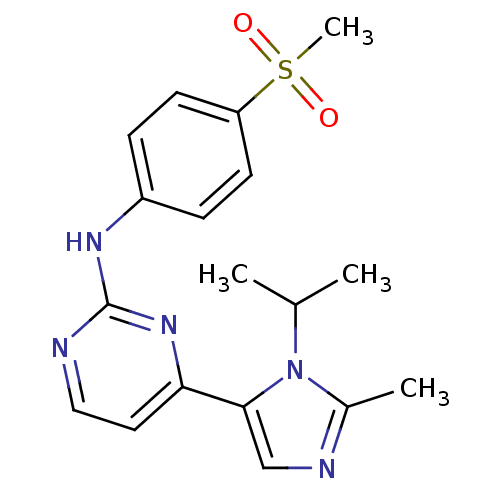 (4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-(me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A2 (unknown origin) using histone H1 and ATP as substrate incubated for 40 mins by kinase-glo luminescence assay | Eur J Med Chem 179: 196-207 (2019) Article DOI: 10.1016/j.ejmech.2019.06.055 BindingDB Entry DOI: 10.7270/Q2833WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50600287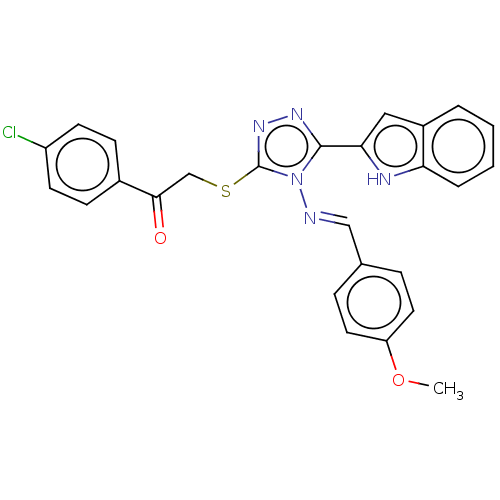 (CHEMBL5178919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128721 BindingDB Entry DOI: 10.7270/Q2HD80QV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of pig brain tubulin polymerization by spectrophotometric analysis | Eur J Med Chem 162: 525-533 (2019) Article DOI: 10.1016/j.ejmech.2018.11.038 BindingDB Entry DOI: 10.7270/Q2CC1436 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50521053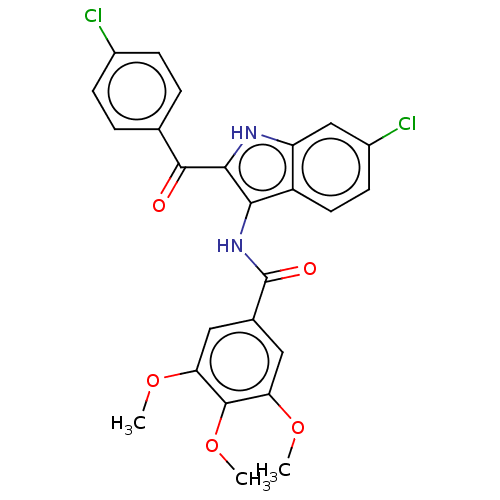 (CHEMBL4578059) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Medical University Curated by ChEMBL | Assay Description Inhibition of pig brain tubulin polymerization by spectrophotometric analysis | Eur J Med Chem 162: 525-533 (2019) Article DOI: 10.1016/j.ejmech.2018.11.038 BindingDB Entry DOI: 10.7270/Q2CC1436 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||