 Found 1252 hits with Last Name = 'zhu' and Initial = 'k'
Found 1252 hits with Last Name = 'zhu' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
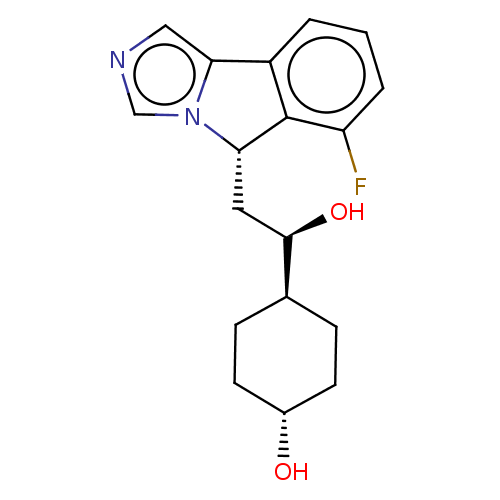
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50503648
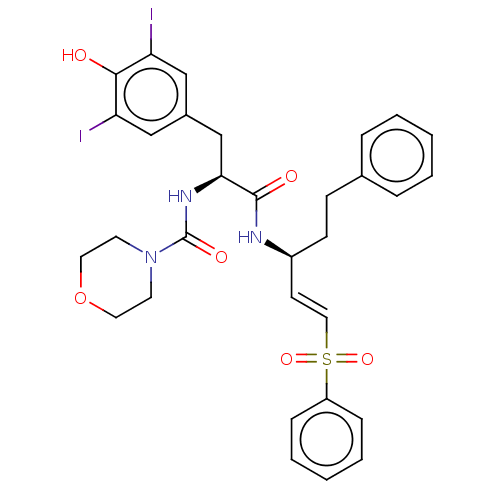
(CHEMBL4474444)Show SMILES Oc1c(I)cc(C[C@H](NC(=O)N2CCOCC2)C(=O)N[C@@H](CCc2ccccc2)\C=C\S(=O)(=O)c2ccccc2)cc1I |r| Show InChI InChI=1S/C31H33I2N3O6S/c32-26-19-23(20-27(33)29(26)37)21-28(35-31(39)36-14-16-42-17-15-36)30(38)34-24(12-11-22-7-3-1-4-8-22)13-18-43(40,41)25-9-5-2-6-10-25/h1-10,13,18-20,24,28,37H,11-12,14-17,21H2,(H,34,38)(H,35,39)/b18-13+/t24-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) using Z-Arg-Arg-AMC as substrate after 5 mins by fluorimetric method |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50503648

(CHEMBL4474444)Show SMILES Oc1c(I)cc(C[C@H](NC(=O)N2CCOCC2)C(=O)N[C@@H](CCc2ccccc2)\C=C\S(=O)(=O)c2ccccc2)cc1I |r| Show InChI InChI=1S/C31H33I2N3O6S/c32-26-19-23(20-27(33)29(26)37)21-28(35-31(39)36-14-16-42-17-15-36)30(38)34-24(12-11-22-7-3-1-4-8-22)13-18-43(40,41)25-9-5-2-6-10-25/h1-10,13,18-20,24,28,37H,11-12,14-17,21H2,(H,34,38)(H,35,39)/b18-13+/t24-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) using Z-Phe-Arg-AMC as substrate after 5 mins by fluorimetric method |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50503647
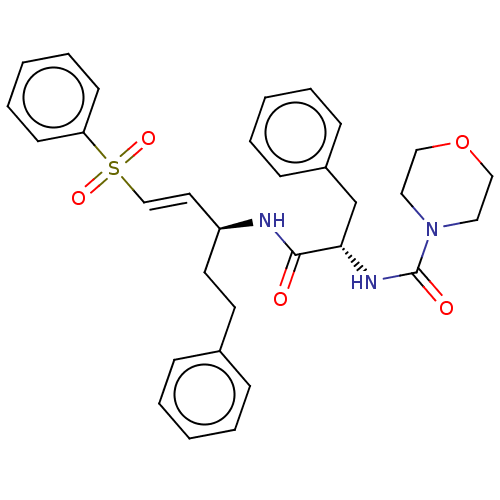
(CHEMBL327624)Show SMILES O=C(N[C@@H](CCc1ccccc1)\C=C\S(=O)(=O)c1ccccc1)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C31H35N3O5S/c35-30(29(24-26-12-6-2-7-13-26)33-31(36)34-19-21-39-22-20-34)32-27(17-16-25-10-4-1-5-11-25)18-23-40(37,38)28-14-8-3-9-15-28/h1-15,18,23,27,29H,16-17,19-22,24H2,(H,32,35)(H,33,36)/b23-18+/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) using Z-Phe-Arg-AMC as substrate after 5 mins by fluorimetric method |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50503647

(CHEMBL327624)Show SMILES O=C(N[C@@H](CCc1ccccc1)\C=C\S(=O)(=O)c1ccccc1)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C31H35N3O5S/c35-30(29(24-26-12-6-2-7-13-26)33-31(36)34-19-21-39-22-20-34)32-27(17-16-25-10-4-1-5-11-25)18-23-40(37,38)28-14-8-3-9-15-28/h1-15,18,23,27,29H,16-17,19-22,24H2,(H,32,35)(H,33,36)/b23-18+/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) using Z-Arg-Arg-AMC as substrate after 5 mins by fluorimetric method |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089
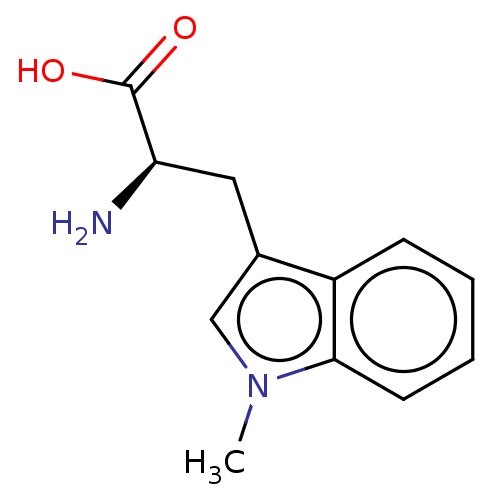
(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of FITC-labeled EZH2 peptide from recombinant N-terminal 6His-tagged EED (residues 81 to 441) (unknown origin) expressed in Escherichia ... |
J Med Chem 57: 9512-21 (2014)
Article DOI: 10.1021/jm501230c
BindingDB Entry DOI: 10.7270/Q270831H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50503646
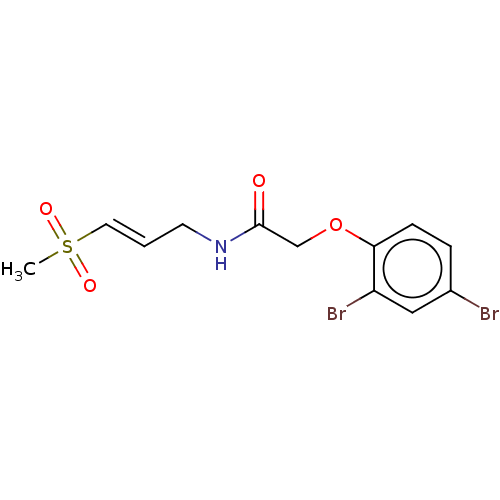
(CHEMBL4564457)Show InChI InChI=1S/C12H13Br2NO4S/c1-20(17,18)6-2-5-15-12(16)8-19-11-4-3-9(13)7-10(11)14/h2-4,6-7H,5,8H2,1H3,(H,15,16)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Competitive inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured at 1 min interval for 20 mins followed by every 15 mins for 2 ... |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50503650

(CHEMBL4516952)Show SMILES C[C@@H](Oc1ccc(Br)cc1Br)C(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C13H15Br2NO4S/c1-9(13(17)16-6-3-7-21(2,18)19)20-12-5-4-10(14)8-11(12)15/h3-5,7-9H,6H2,1-2H3,(H,16,17)/b7-3+/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured at 1 min interval for 20 mins followed by every 15 mins for 2 hrs by fluor... |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50503649

(CHEMBL4476113)Show SMILES CC1(CC1)c1cc(NCC(=O)N2CCN(CC2)C2CN(C2)C(=O)C=C)c(O)cc1Cl Show InChI InChI=1S/C22H29ClN4O3/c1-3-20(29)27-13-15(14-27)25-6-8-26(9-7-25)21(30)12-24-18-10-16(22(2)4-5-22)17(23)11-19(18)28/h3,10-11,15,24,28H,1,4-9,12-14H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Houston
Curated by ChEMBL
| Assay Description
Inhibition of KRAS G12C mutant (unknown origin) |
Bioorg Med Chem Lett 29: 36-39 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.019
BindingDB Entry DOI: 10.7270/Q2028VTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50462572

(CHEMBL4249337)Show SMILES Nc1nc2cc(CC[C@H]3C[C@H]([C@H](O)[C@@H]3O)n3ccc4c(N)ncnc34)ccc2cc1Br |r| Show InChI InChI=1S/C22H23BrN6O2/c23-15-8-12-3-1-11(7-16(12)28-21(15)25)2-4-13-9-17(19(31)18(13)30)29-6-5-14-20(24)26-10-27-22(14)29/h1,3,5-8,10,13,17-19,30-31H,2,4,9H2,(H2,25,28)(H2,24,26,27)/t13-,17+,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal FLAG-tagged PRMT5 expressed in Sf9 insect cells using histone H2A as peptide after 120 mins in presence of... |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM415557

(US10428104, Example 88)Show SMILES Nc1ncnc2n(ccc12)[C@@H]1O[C@H]([C@H](O)c2ccc(Cl)c(F)c2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H16ClFN4O4/c18-9-2-1-7(5-10(9)19)11(24)14-12(25)13(26)17(27-14)23-4-3-8-15(20)21-6-22-16(8)23/h1-6,11-14,17,24-26H,(H2,20,21,22)/t11-,12+,13-,14-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin) |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
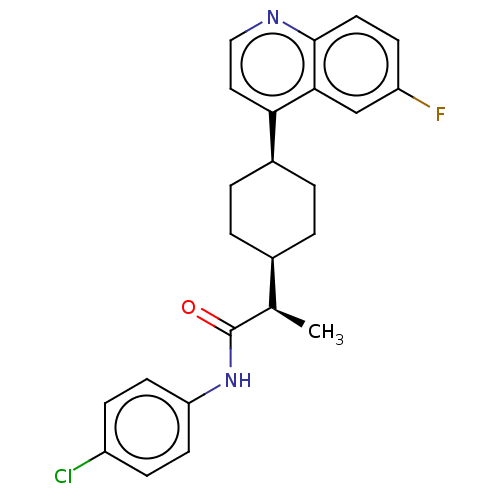
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in baculovirus expression system using poly (Glu-Tyr) 4:1 as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02005
BindingDB Entry DOI: 10.7270/Q2X06BR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in baculovirus expression system using poly (Glu-Tyr) 4:1 as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02005
BindingDB Entry DOI: 10.7270/Q2X06BR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653019
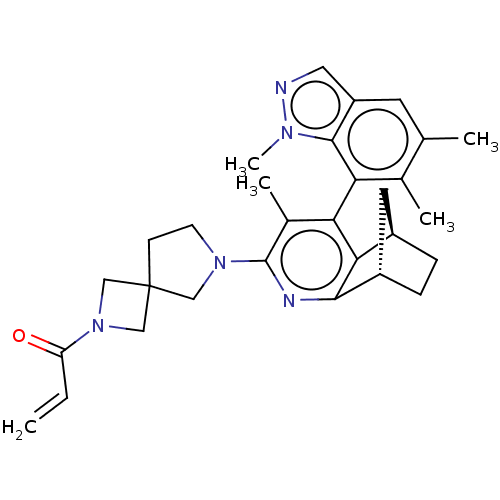
((M)-1-(6- ((1S,8R)-5- methyl-6-(1,5,6- trimethyl-1...)Show SMILES Cc1cc2cnn(C)c2c(c1C)-c1c2[C@@H]3CC[C@@H](C3)c2nc(N2CCC3(CN(C3)C(=O)C=C)C2)c1C |r,wU:17.19,14.20,(5.55,-3.51,;4.22,-4.28,;4.22,-5.82,;2.88,-6.59,;2.56,-8.1,;1.03,-8.26,;.41,-6.85,;-1.08,-6.45,;1.55,-5.82,;1.55,-4.28,;2.88,-3.51,;2.88,-1.97,;.22,-3.51,;-1.12,-4.28,;-1.12,-5.82,;-2.45,-6.59,;-3.78,-5.82,;-3.78,-4.28,;-2.3,-4.68,;-2.45,-3.51,;-2.45,-1.97,;-1.12,-1.2,;-1.12,.34,;.13,1.24,;-.35,2.71,;-1.89,2.71,;-1.49,4.2,;-2.98,4.59,;-3.37,3.11,;-4.06,5.68,;-5.55,5.28,;-3.67,7.17,;-4.75,8.26,;-2.36,1.24,;.22,-1.97,;1.55,-1.2,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50609343
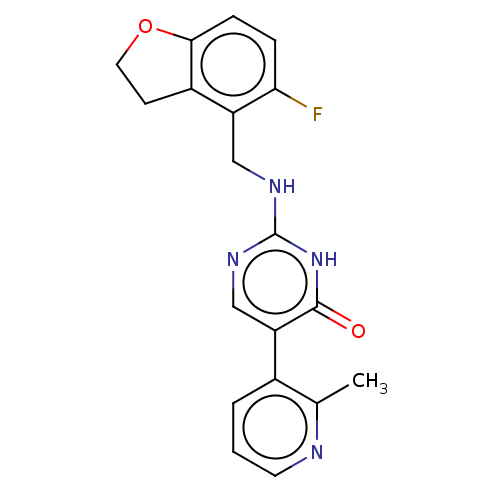
(CHEMBL5269445)Show SMILES [2H]C([2H])(Nc1ncc(-c2cccnc2C)c(=O)[nH]1)c1c2CCOc2ccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM652997
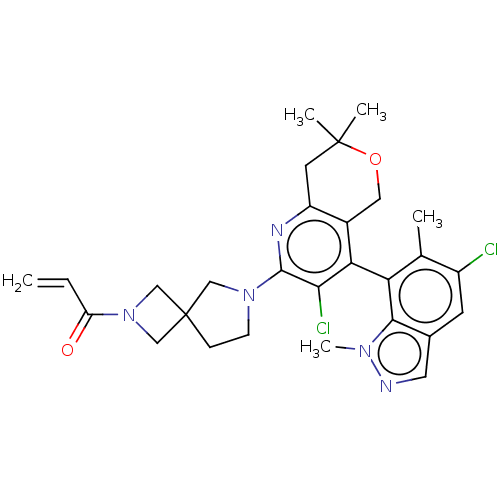
(1-(6-(3-chloro- 4-(1,6-dimethyl- 1H-indazol-7- yl)...)Show SMILES Cc1c(Cl)cc2cnn(C)c2c1-c1c(Cl)c(nc2CC(C)(C)OCc12)N1CCC2(CN(C2)C(=O)C=C)C1 |(2.88,-1.97,;2.88,-3.51,;4.22,-4.28,;5.55,-3.51,;4.22,-5.82,;2.88,-6.59,;2.56,-8.1,;1.03,-8.26,;.41,-6.85,;-1.08,-6.45,;1.55,-5.82,;1.55,-4.28,;.22,-3.51,;.22,-1.97,;1.55,-1.2,;-1.12,-1.2,;-2.45,-1.97,;-2.45,-3.51,;-3.78,-4.28,;-3.78,-5.82,;-5.12,-6.59,;-3.78,-7.36,;-2.45,-6.59,;-1.12,-5.82,;-1.12,-4.28,;-1.12,.34,;.13,1.24,;-.35,2.71,;-1.89,2.71,;-1.49,4.2,;-2.98,4.59,;-3.37,3.11,;-4.06,5.68,;-5.55,5.28,;-3.67,7.17,;-4.75,8.26,;-2.36,1.24,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology
| Assay Description
This spectrophotometric assay is based on the reaction of
5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a
colored product. Sh... |
Chem Biol Drug Des 88: 889-898 (2016)
Article DOI: 10.1111/cbdd.12822
BindingDB Entry DOI: 10.7270/Q2TX3D65 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM652949
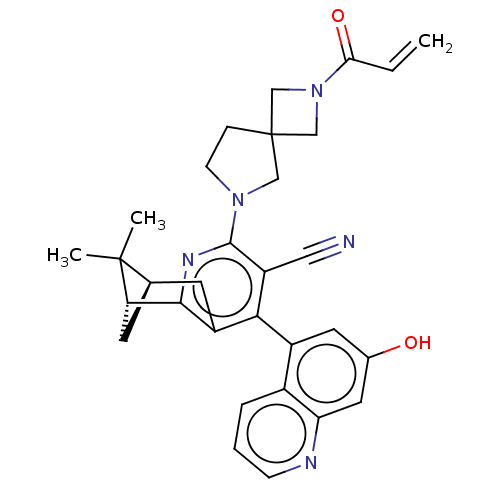
((1R,9R)-6-(7- hydroxy-5- quinolinyl)- 10,10-dimeth...)Show SMILES CC1(C)[C@@H]2C[C@H]1c1nc(N3CCC4(CN(C4)C(=O)C=C)C3)c(C#N)c(c1C2)-c1cc(O)cc2ncccc12 |r,wU:5.4,3.3,(-5.12,-4.96,;-3.58,-4.96,;-4.35,-6.29,;-2.25,-5.73,;-2.25,-4.19,;-3.58,-3.42,;-2.25,-2.65,;-2.25,-1.11,;-.92,-.34,;-.92,1.2,;.33,2.11,;-.15,3.57,;-1.69,3.57,;-1.29,5.06,;-2.78,5.46,;-3.17,3.97,;-3.87,6.55,;-3.47,8.04,;-5.35,6.15,;-5.75,4.66,;-2.16,2.11,;.42,-1.11,;1.75,-.34,;3.08,.43,;.42,-2.65,;-.92,-3.42,;-.92,-4.96,;1.75,-3.42,;3.08,-2.65,;4.42,-3.42,;5.75,-2.65,;4.42,-4.96,;3.08,-5.73,;3.08,-7.27,;1.75,-8.04,;.42,-7.27,;.42,-5.73,;1.75,-4.96,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM178166
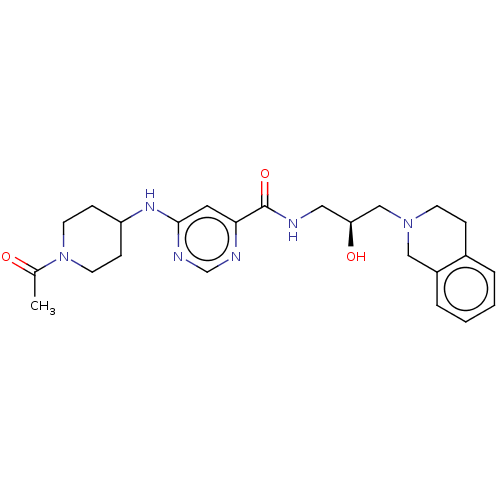
(US10307413, Compound 208 | US10391089, Compound 20...)Show SMILES CC(=O)N1CCC(CC1)Nc1cc(ncn1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C24H32N6O3/c1-17(31)30-10-7-20(8-11-30)28-23-12-22(26-16-27-23)24(33)25-13-21(32)15-29-9-6-18-4-2-3-5-19(18)14-29/h2-5,12,16,20-21,32H,6-11,13-15H2,1H3,(H,25,33)(H,26,27,28)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin) |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM178166

(US10307413, Compound 208 | US10391089, Compound 20...)Show SMILES CC(=O)N1CCC(CC1)Nc1cc(ncn1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C24H32N6O3/c1-17(31)30-10-7-20(8-11-30)28-23-12-22(26-16-27-23)24(33)25-13-21(32)15-29-9-6-18-4-2-3-5-19(18)14-29/h2-5,12,16,20-21,32H,6-11,13-15H2,1H3,(H,25,33)(H,26,27,28)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin)/MEP50 (unknown origin) using histone H4 as substrate preincubated for 60 mins in presence of enzyme and SAM |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562497
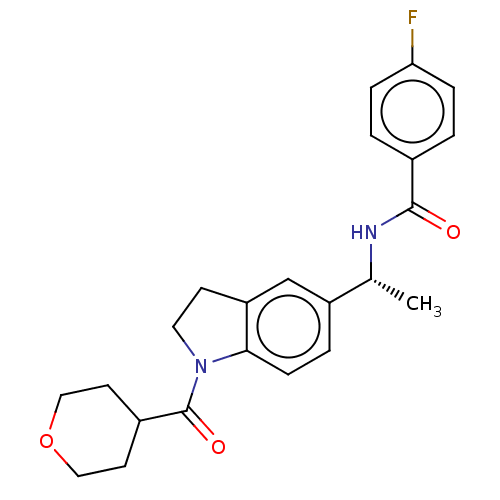
(CHEMBL4778760)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCc2c1)C(=O)C1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653472
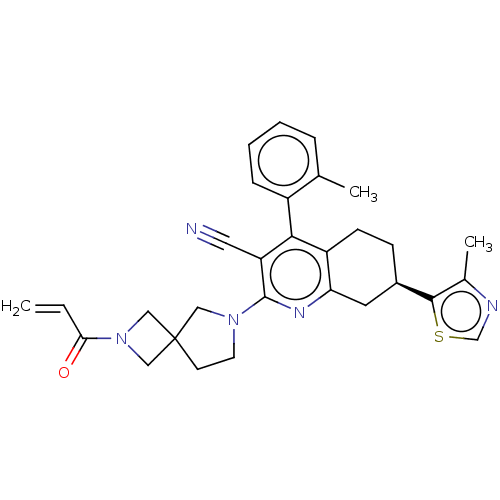
((M)-(7R)-4-(2-methylphenyl)-7-(4- methyl-1,3-thiaz...)Show SMILES Cc1ncsc1[C@@H]1CCc2c(C1)nc(N1CCC3(CN(C3)C(=O)C=C)C1)c(C#N)c2-c1ccccc1C |r,wD:6.6,(5.35,-3.84,;4.95,-5.33,;5.98,-6.47,;5.21,-7.81,;3.7,-7.49,;3.54,-5.96,;2.21,-5.19,;.88,-5.96,;-.46,-5.19,;-.46,-3.65,;.88,-2.88,;2.21,-3.65,;.88,-1.34,;-.46,-.57,;-.46,.97,;.79,1.88,;.31,3.34,;-1.23,3.34,;-.83,4.83,;-2.32,5.23,;-2.71,3.74,;-3.4,6.32,;-3.01,7.81,;-4.89,5.92,;-5.98,7.01,;-1.7,1.88,;-1.79,-1.34,;-3.12,-.57,;-4.46,.2,;-1.79,-2.88,;-3.12,-3.65,;-4.46,-2.88,;-5.79,-3.65,;-5.79,-5.19,;-4.46,-5.96,;-3.12,-5.19,;-1.79,-5.96,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50503687
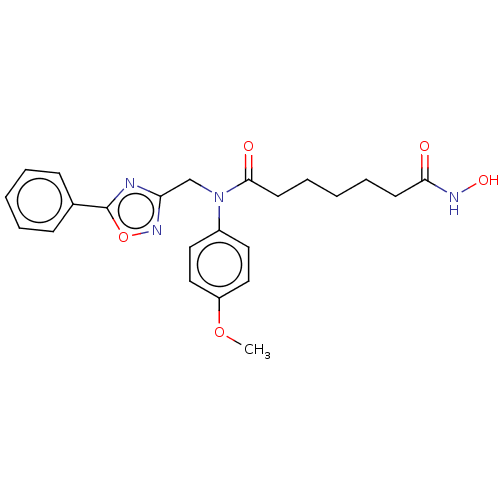
(CHEMBL4465444)Show SMILES COc1ccc(cc1)N(Cc1noc(n1)-c1ccccc1)C(=O)CCCCCC(=O)NO Show InChI InChI=1S/C23H26N4O5/c1-31-19-14-12-18(13-15-19)27(22(29)11-7-3-6-10-21(28)25-30)16-20-24-23(32-26-20)17-8-4-2-5-9-17/h2,4-5,8-9,12-15,30H,3,6-7,10-11,16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using Boc-Lys (Ac)-AMC as substrate measured after 60 mins by ELISA |
Bioorg Med Chem Lett 29: 15-21 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.027
BindingDB Entry DOI: 10.7270/Q2KW5K9T |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529897

(CHEMBL4468383)Show SMILES O[C@@H](CNC(=O)c1ccc2CN(CCc2c1)C(=O)c1ccc(Br)cc1)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C29H30BrN3O3/c30-26-9-7-21(8-10-26)29(36)33-14-12-22-15-23(5-6-25(22)18-33)28(35)31-16-27(34)19-32-13-11-20-3-1-2-4-24(20)17-32/h1-10,15,27,34H,11-14,16-19H2,(H,31,35)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529897

(CHEMBL4468383)Show SMILES O[C@@H](CNC(=O)c1ccc2CN(CCc2c1)C(=O)c1ccc(Br)cc1)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C29H30BrN3O3/c30-26-9-7-21(8-10-26)29(36)33-14-12-22-15-23(5-6-25(22)18-33)28(35)31-16-27(34)19-32-13-11-20-3-1-2-4-24(20)17-32/h1-10,15,27,34H,11-14,16-19H2,(H,31,35)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM652946
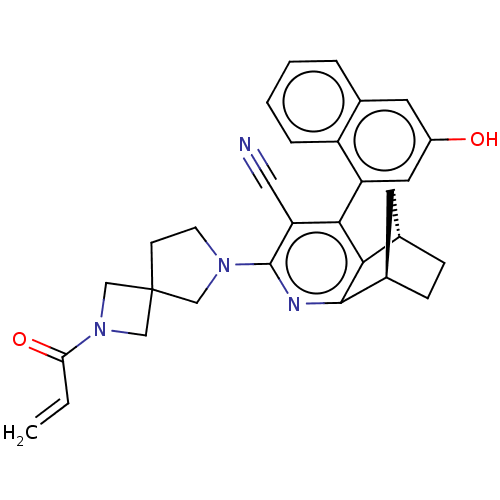
((P)-(1R,8S)-6- (3-hydroxy-1- naphthalenyl)-4- (2-(...)Show SMILES Oc1cc(-c2c3[C@H]4CC[C@H](C4)c3nc(N3CCC4(CN(C4)C(=O)C=C)C3)c2C#N)c2ccccc2c1 |r,wD:9.9,6.10,(6.1,-2.65,;4.76,-3.42,;3.43,-2.65,;2.1,-3.42,;.76,-2.65,;-.57,-3.42,;-.57,-4.96,;-1.91,-5.73,;-3.24,-4.96,;-3.24,-3.42,;-1.75,-3.81,;-1.91,-2.65,;-1.91,-1.11,;-.57,-.34,;-.57,1.2,;.67,2.11,;.2,3.57,;-1.34,3.57,;-.94,5.06,;-2.43,5.46,;-2.83,3.97,;-3.52,6.55,;-3.12,8.04,;-5.01,6.15,;-6.1,7.24,;-1.82,2.11,;.76,-1.11,;2.1,-.34,;3.43,.43,;2.1,-4.96,;.76,-5.73,;.76,-7.27,;2.1,-8.04,;3.43,-7.27,;3.43,-5.73,;4.76,-4.96,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653301
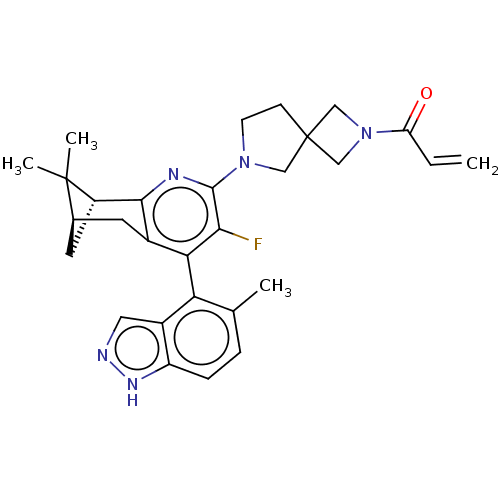
(1-(6-((1R,9R)-5-fluoro- 10,10-dimethyl-6-(5- methy...)Show SMILES Cc1ccc2[nH]ncc2c1-c1c(F)c(nc2[C@@H]3C[C@H](Cc12)C3(C)C)N1CCC2(CN(C2)C(=O)C=C)C1 |r,wU:16.18,18.19,(3.75,-1.43,;3.75,-2.97,;5.08,-3.74,;5.08,-5.28,;3.75,-6.05,;3.43,-7.55,;1.9,-7.71,;1.27,-6.31,;2.42,-5.28,;2.42,-3.74,;1.08,-2.97,;1.08,-1.43,;2.42,-.66,;-.25,-.66,;-1.58,-1.43,;-1.58,-2.97,;-2.92,-3.74,;-1.58,-4.51,;-1.58,-6.05,;-.25,-5.28,;-.25,-3.74,;-2.92,-5.28,;-4.46,-5.28,;-3.32,-6.76,;-.25,.88,;1,1.79,;.52,3.25,;-1.02,3.25,;-.62,4.74,;-2.11,5.14,;-2.51,3.65,;-3.2,6.23,;-2.8,7.71,;-4.69,5.83,;-5.08,4.34,;-1.5,1.79,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529901

(CHEMBL4568997)Show SMILES OC(CNC(=O)c1ccc2CN(CCc2c1)C(=O)c1ccc(Br)cc1)CN1CCc2ccccc2C1 Show InChI InChI=1S/C29H30BrN3O3/c30-26-9-7-21(8-10-26)29(36)33-14-12-22-15-23(5-6-25(22)18-33)28(35)31-16-27(34)19-32-13-11-20-3-1-2-4-24(20)17-32/h1-10,15,27,34H,11-14,16-19H2,(H,31,35) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529900

(CHEMBL4473655)Show SMILES CN(C)c1ccc(cc1)C(=O)N1CCc2cc(ccc2C1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C31H36N4O3/c1-33(2)28-11-9-23(10-12-28)31(38)35-16-14-24-17-25(7-8-27(24)20-35)30(37)32-18-29(36)21-34-15-13-22-5-3-4-6-26(22)19-34/h3-12,17,29,36H,13-16,18-21H2,1-2H3,(H,32,37)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50503688
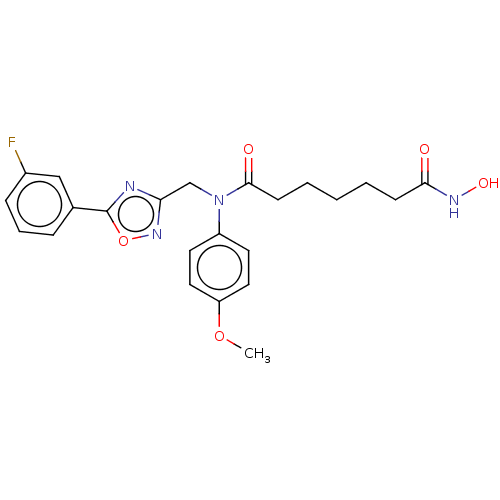
(CHEMBL4545154)Show SMILES COc1ccc(cc1)N(Cc1noc(n1)-c1cccc(F)c1)C(=O)CCCCCC(=O)NO Show InChI InChI=1S/C23H25FN4O5/c1-32-19-12-10-18(11-13-19)28(22(30)9-4-2-3-8-21(29)26-31)15-20-25-23(33-27-20)16-6-5-7-17(24)14-16/h5-7,10-14,31H,2-4,8-9,15H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using Boc-Lys (Ac)-AMC as substrate measured after 60 mins by ELISA |
Bioorg Med Chem Lett 29: 15-21 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.027
BindingDB Entry DOI: 10.7270/Q2KW5K9T |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653277
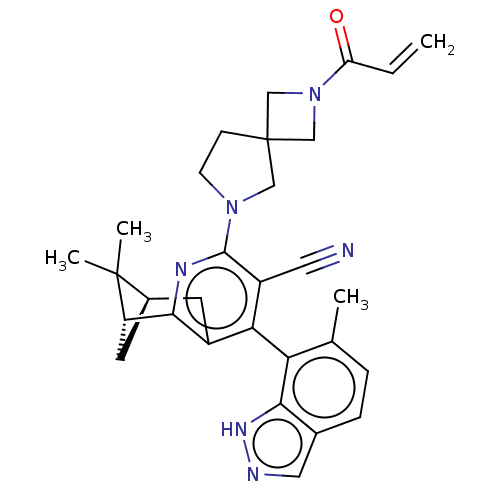
((1R,9R)-10,10-dimethyl- 6-(6-methyl-1H-indazol- 7-...)Show SMILES Cc1ccc2cn[nH]c2c1-c1c2C[C@H]3C[C@@H](c2nc(N2CCC4(CN(C4)C(=O)C=C)C2)c1C#N)C3(C)C |r,wU:15.16,13.15,(3.75,-1.43,;3.75,-2.97,;5.08,-3.74,;5.08,-5.28,;3.75,-6.05,;3.43,-7.55,;1.9,-7.71,;1.27,-6.31,;2.42,-5.28,;2.42,-3.74,;1.08,-2.97,;-.25,-3.74,;-.25,-5.28,;-1.58,-6.05,;-1.58,-4.51,;-2.92,-3.74,;-1.58,-2.97,;-1.58,-1.43,;-.25,-.66,;-.25,.88,;1,1.79,;.52,3.25,;-1.02,3.25,;-.62,4.74,;-2.11,5.14,;-2.51,3.65,;-3.2,6.23,;-2.8,7.71,;-4.69,5.83,;-5.08,4.34,;-1.5,1.79,;1.08,-1.43,;2.42,-.66,;3.75,.11,;-2.92,-5.28,;-4.46,-5.28,;-3.32,-6.76,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529901

(CHEMBL4568997)Show SMILES OC(CNC(=O)c1ccc2CN(CCc2c1)C(=O)c1ccc(Br)cc1)CN1CCc2ccccc2C1 Show InChI InChI=1S/C29H30BrN3O3/c30-26-9-7-21(8-10-26)29(36)33-14-12-22-15-23(5-6-25(22)18-33)28(35)31-16-27(34)19-32-13-11-20-3-1-2-4-24(20)17-32/h1-10,15,27,34H,11-14,16-19H2,(H,31,35) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529900

(CHEMBL4473655)Show SMILES CN(C)c1ccc(cc1)C(=O)N1CCc2cc(ccc2C1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C31H36N4O3/c1-33(2)28-11-9-23(10-12-28)31(38)35-16-14-24-17-25(7-8-27(24)20-35)30(37)32-18-29(36)21-34-15-13-22-5-3-4-6-26(22)19-34/h3-12,17,29,36H,13-16,18-21H2,1-2H3,(H,32,37)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50503686
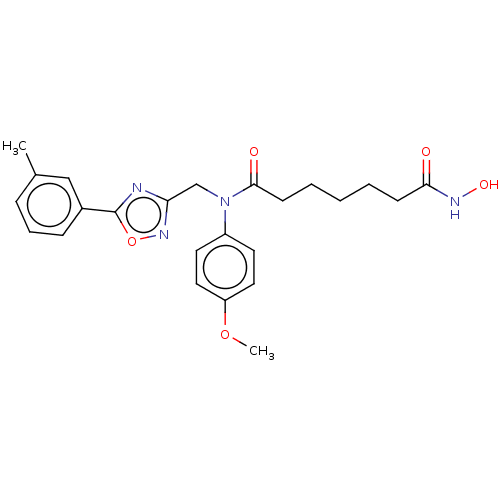
(CHEMBL4449889)Show SMILES COc1ccc(cc1)N(Cc1noc(n1)-c1cccc(C)c1)C(=O)CCCCCC(=O)NO Show InChI InChI=1S/C24H28N4O5/c1-17-7-6-8-18(15-17)24-25-21(27-33-24)16-28(19-11-13-20(32-2)14-12-19)23(30)10-5-3-4-9-22(29)26-31/h6-8,11-15,31H,3-5,9-10,16H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using Boc-Lys (Ac)-AMC as substrate measured after 60 mins by ELISA |
Bioorg Med Chem Lett 29: 15-21 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.027
BindingDB Entry DOI: 10.7270/Q2KW5K9T |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653092
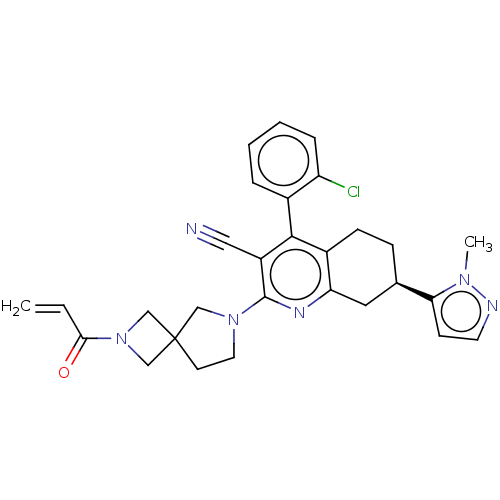
((7R)-4-(2-chlorophenyl)-7- (1-methyl-1H-pyrazol-5-...)Show SMILES Cn1nccc1[C@@H]1CCc2c(C1)nc(N1CCC3(CN(C3)C(=O)C=C)C1)c(C#N)c2-c1ccccc1Cl |r,wU:6.6,(-2.52,-8.19,;-3.61,-7.1,;-5.12,-7.42,;-5.89,-6.09,;-4.86,-4.94,;-3.45,-5.57,;-2.12,-4.8,;-.78,-5.57,;.55,-4.8,;.55,-3.26,;-.78,-2.49,;-2.12,-3.26,;-.78,-.95,;.55,-.18,;.55,1.36,;1.8,2.26,;1.32,3.73,;-.22,3.73,;.18,5.22,;-1.31,5.61,;-1.71,4.13,;-2.4,6.7,;-2,8.19,;-3.88,6.31,;-4.28,4.82,;-.69,2.26,;1.93,-.92,;3.27,-.15,;4.6,.63,;1.89,-2.49,;3.22,-3.26,;3.22,-4.8,;4.55,-5.57,;5.89,-4.8,;5.89,-3.26,;4.55,-2.49,;4.55,-.95,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653165
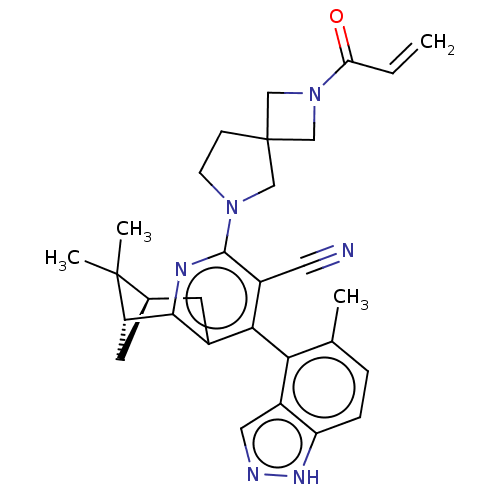
((6R,8R)-2-(2-acryloyl-2,6- diazaspiro[3.4]octan-6-...)Show SMILES Cc1ccc2[nH]ncc2c1-c1c2C[C@H]3C[C@@H](c2nc(N2CCC4(CN(C4)C(=O)C=C)C2)c1C#N)C3(C)C |r,wU:15.16,13.15,(3.75,-1.43,;3.75,-2.97,;5.08,-3.74,;5.08,-5.28,;3.75,-6.05,;3.43,-7.55,;1.9,-7.71,;1.27,-6.31,;2.42,-5.28,;2.42,-3.74,;1.08,-2.97,;-.25,-3.74,;-.25,-5.28,;-1.58,-6.05,;-1.58,-4.51,;-2.92,-3.74,;-1.58,-2.97,;-1.58,-1.43,;-.25,-.66,;-.25,.88,;1,1.79,;.52,3.25,;-1.02,3.25,;-.62,4.74,;-2.11,5.14,;-2.51,3.65,;-3.2,6.23,;-2.8,7.71,;-4.69,5.83,;-5.08,4.34,;-1.5,1.79,;1.08,-1.43,;2.42,-.66,;3.75,.11,;-2.92,-5.28,;-4.41,-5.68,;-2.92,-6.82,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653180
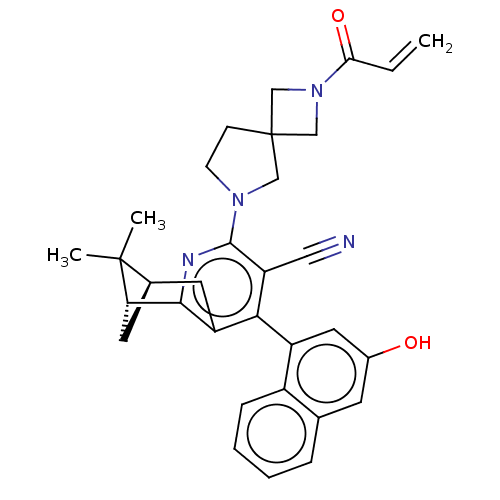
((1R,9R)-6-(3-hydroxy-1- naphthalenyl)-10,10- dimet...)Show SMILES CC1(C)[C@@H]2C[C@H]1c1nc(N3CCC4(CN(C4)C(=O)C=C)C3)c(C#N)c(c1C2)-c1cc(O)cc2ccccc12 |r,wU:3.3,5.4,(-5.07,-5.35,;-3.58,-4.96,;-3.58,-6.5,;-2.25,-5.73,;-2.25,-4.19,;-3.58,-3.42,;-2.25,-2.65,;-2.25,-1.11,;-.92,-.34,;-.92,1.2,;.33,2.11,;-.15,3.57,;-1.69,3.57,;-1.29,5.06,;-2.78,5.46,;-3.17,3.97,;-3.87,6.55,;-3.47,8.04,;-5.35,6.15,;-5.75,4.66,;-2.16,2.11,;.42,-1.11,;1.75,-.34,;3.08,.43,;.42,-2.65,;-.92,-3.42,;-.92,-4.96,;1.75,-3.42,;3.08,-2.65,;4.42,-3.42,;5.75,-2.65,;4.42,-4.96,;3.08,-5.73,;3.08,-7.27,;1.75,-8.04,;.42,-7.27,;.42,-5.73,;1.75,-4.96,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653162
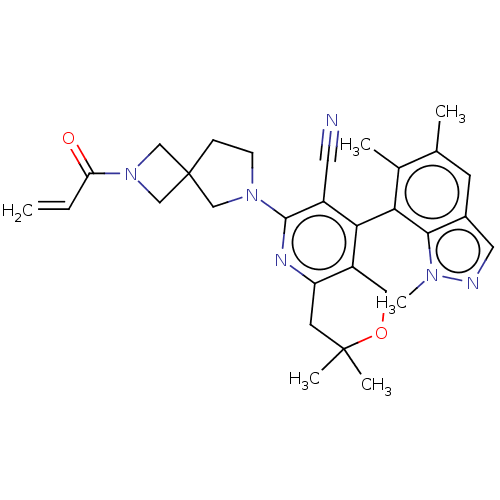
(7,7-dimethyl-2-(2-(2- propenoyl)-2,6- diazaspiro[3...)Show SMILES Cc1cc2cnn(C)c2c(c1C)-c1c2COC(C)(C)Cc2nc(N2CCC3(CN(C3)C(=O)C=C)C2)c1C#N |(5.75,-2.97,;4.42,-3.74,;4.42,-5.28,;3.08,-6.05,;2.76,-7.55,;1.23,-7.71,;.61,-6.31,;-.88,-5.91,;1.75,-5.28,;1.75,-3.74,;3.08,-2.97,;3.08,-1.43,;.42,-2.97,;-.92,-3.74,;-.92,-5.28,;-2.25,-6.05,;-3.58,-5.28,;-5.07,-5.68,;-3.58,-6.82,;-3.58,-3.74,;-2.25,-2.97,;-2.25,-1.43,;-.92,-.66,;-.92,.88,;.33,1.79,;-.15,3.25,;-1.69,3.25,;-1.29,4.74,;-2.78,5.14,;-3.17,3.65,;-3.87,6.23,;-3.47,7.71,;-5.35,5.83,;-5.75,4.34,;-2.16,1.79,;.42,-1.43,;1.75,-.66,;3.08,.11,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529896

(CHEMBL4444027)Show SMILES COc1ccc(cc1)C(=O)N1CCc2cc(ccc2C1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C30H33N3O4/c1-37-28-10-8-22(9-11-28)30(36)33-15-13-23-16-24(6-7-26(23)19-33)29(35)31-17-27(34)20-32-14-12-21-4-2-3-5-25(21)18-32/h2-11,16,27,34H,12-15,17-20H2,1H3,(H,31,35)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50529896

(CHEMBL4444027)Show SMILES COc1ccc(cc1)C(=O)N1CCc2cc(ccc2C1)C(=O)NC[C@H](O)CN1CCc2ccccc2C1 |r| Show InChI InChI=1S/C30H33N3O4/c1-37-28-10-8-22(9-11-28)30(36)33-15-13-23-16-24(6-7-26(23)19-33)29(35)31-17-27(34)20-32-14-12-21-4-2-3-5-25(21)18-32/h2-11,16,27,34H,12-15,17-20H2,1H3,(H,31,35)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal FLAG-tagged PRMT5 (2 to end residues)/human N-terminal His-tagged MEP50 (2 to end residues) expressed in H... |
Eur J Med Chem 164: 317-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.065
BindingDB Entry DOI: 10.7270/Q2RR22Q1 |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653336
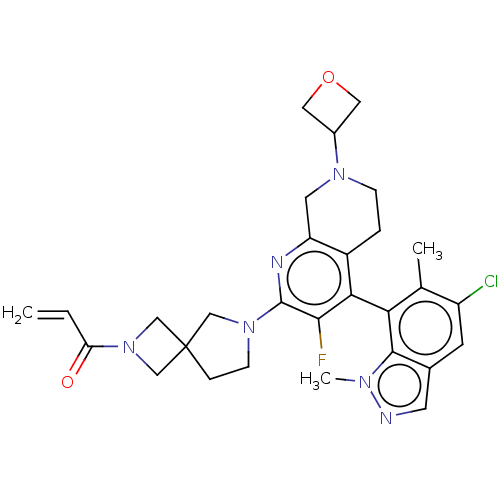
((M)-1-(6-(4-(5-chloro- 1,6-dimethyl-1H- indazol-7-...)Show SMILES Cc1c(Cl)cc2cnn(C)c2c1-c1c(F)c(nc2CN(CCc12)C1COC1)N1CCC2(CN(C2)C(=O)C=C)C1 |(3.61,-1.43,;3.61,-2.97,;4.94,-3.74,;6.28,-2.97,;4.94,-5.28,;3.61,-6.05,;3.29,-7.55,;1.76,-7.71,;1.13,-6.31,;-.36,-5.91,;2.28,-5.28,;2.28,-3.74,;.94,-2.97,;.94,-1.43,;2.28,-.66,;-.39,-.66,;-1.72,-1.43,;-1.72,-2.97,;-3.06,-3.74,;-3.06,-5.28,;-1.72,-6.05,;-.39,-5.28,;-.39,-3.74,;-4.39,-6.05,;-5.88,-5.65,;-6.28,-7.14,;-4.79,-7.53,;-.39,.88,;.86,1.79,;.38,3.25,;-1.16,3.25,;-.76,4.74,;-2.25,5.14,;-2.65,3.65,;-3.34,6.23,;-2.94,7.71,;-4.83,5.83,;-5.22,4.34,;-1.64,1.79,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]/Son of sevenless homolog 1 [564-1049]
(Homo sapiens (Human)) | BDBM653305
((M)-1-(6-((1R,9R)-5- fluoro-6-(3-hydroxy-1- naphth...)Show SMILES CC1(C)[C@@H]2C[C@H]1c1nc(N3CCC4(CN(C4)C(=O)C=C)C3)c(F)c(c1C2)-c1cc(O)cc2ccccc12 |r,wU:5.4,3.3,(-5.12,-4.96,;-3.58,-4.96,;-3.98,-6.44,;-2.25,-5.73,;-2.25,-4.19,;-3.58,-3.42,;-2.25,-2.65,;-2.25,-1.11,;-.92,-.34,;-.92,1.2,;.33,2.11,;-.15,3.57,;-1.69,3.57,;-1.29,5.06,;-2.78,5.46,;-3.17,3.97,;-3.87,6.55,;-3.47,8.04,;-5.35,6.15,;-5.75,4.66,;-2.16,2.11,;.42,-1.11,;1.75,-.34,;.42,-2.65,;-.92,-3.42,;-.92,-4.96,;1.75,-3.42,;3.08,-2.65,;4.42,-3.42,;5.75,-2.65,;4.42,-4.96,;3.08,-5.73,;3.08,-7.27,;1.75,-8.04,;.42,-7.27,;.42,-5.73,;1.75,-4.96,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data