

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibitory constant against human adenosne A3 receptor | J Med Chem 50: 65-73 (2007) Article DOI: 10.1021/jm061045z BindingDB Entry DOI: 10.7270/Q2TD9Z40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibitory constant aganist human adenosine A1 receptor | J Med Chem 50: 65-73 (2007) Article DOI: 10.1021/jm061045z BindingDB Entry DOI: 10.7270/Q2TD9Z40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50202002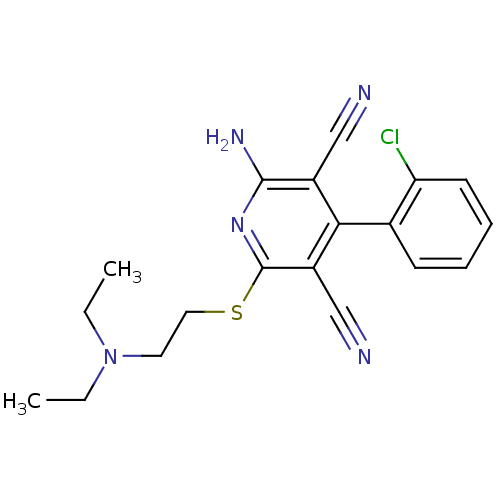 (2-amino-4-(2-chlorophenyl)-6-(2-(diethylamino)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibitory constant aganist human adenosine A1 receptor | J Med Chem 50: 65-73 (2007) Article DOI: 10.1021/jm061045z BindingDB Entry DOI: 10.7270/Q2TD9Z40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibitory constant against human adenosine A2a receptor | J Med Chem 50: 65-73 (2007) Article DOI: 10.1021/jm061045z BindingDB Entry DOI: 10.7270/Q2TD9Z40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50326058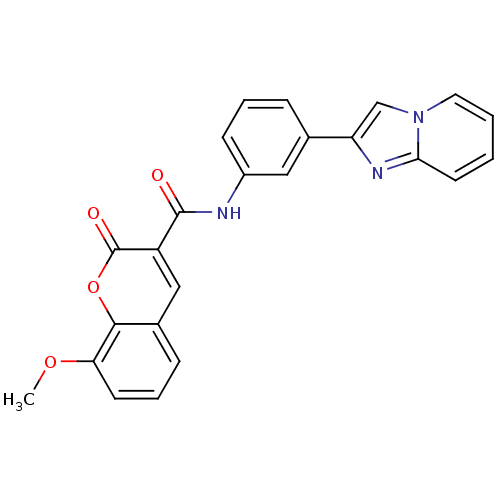 (CHEMBL1242213 | N-(3-(imidazo[1,2-a]pyridin-2-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of mature Caspase3 | Nat Chem Biol 6: 179-188 (2010) Article DOI: 10.1038/nchembio.318 BindingDB Entry DOI: 10.7270/Q25Q4W9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||