 Found 15 hits for monomerid = 50225216,50225218,50225219,50225222,50225224,50225225,50225226,50225228,50225230,50225231
Found 15 hits for monomerid = 50225216,50225218,50225219,50225222,50225224,50225225,50225226,50225228,50225230,50225231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transthyretin
(Homo sapiens (Human)) | BDBM50225216
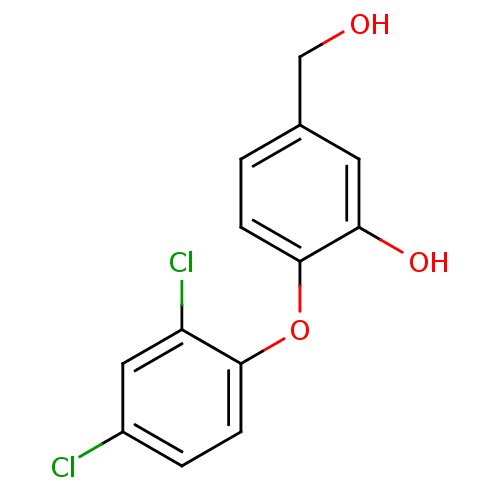
(2-(2,4-dichlorophenoxy)-5-(hydroxymethyl)phenol | ...)Show InChI InChI=1S/C13H10Cl2O3/c14-9-2-4-12(10(15)6-9)18-13-3-1-8(7-16)5-11(13)17/h1-6,16-17H,7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to transthyretin at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225231

(3-methoxy-4-phenoxybenzoic acid | CHEMBL392872)Show InChI InChI=1S/C14H12O4/c1-17-13-9-10(14(15)16)7-8-12(13)18-11-5-3-2-4-6-11/h2-9H,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transthyretin
(Homo sapiens (Human)) | BDBM50225216

(2-(2,4-dichlorophenoxy)-5-(hydroxymethyl)phenol | ...)Show InChI InChI=1S/C13H10Cl2O3/c14-9-2-4-12(10(15)6-9)18-13-3-1-8(7-16)5-11(13)17/h1-6,16-17H,7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225219
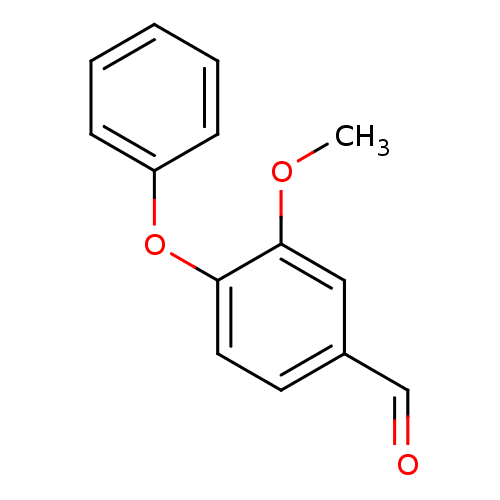
(3-methoxy-4-phenoxybenzaldehyde | CHEMBL241453)Show InChI InChI=1S/C14H12O3/c1-16-14-9-11(10-15)7-8-13(14)17-12-5-3-2-4-6-12/h2-10H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 88 | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to transthyretin at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225224
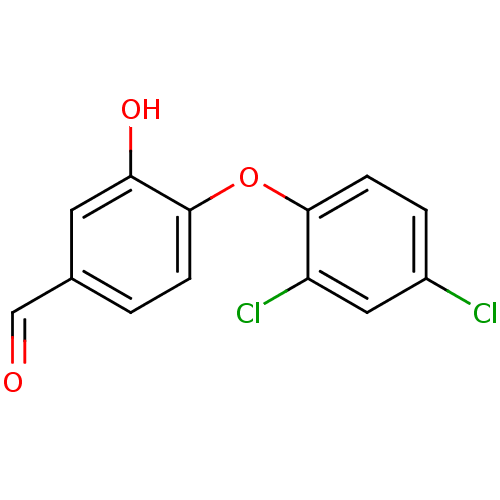
(4-(2,4-dichlorophenoxy)-3-hydroxybenzaldehyde | CH...)Show InChI InChI=1S/C13H8Cl2O3/c14-9-2-4-12(10(15)6-9)18-13-3-1-8(7-16)5-11(13)17/h1-7,17H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 13.9 | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to transthyretin at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transthyretin
(Homo sapiens (Human)) | BDBM50225222
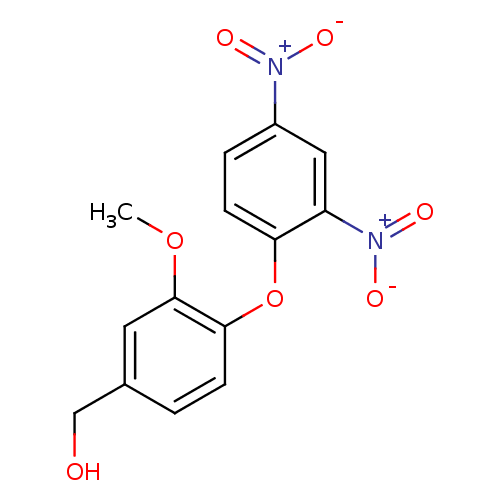
((4-(2,4-dinitrophenoxy)-3-methoxyphenyl)methanol |...)Show SMILES COc1cc(CO)ccc1Oc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C14H12N2O7/c1-22-14-6-9(8-17)2-4-13(14)23-12-5-3-10(15(18)19)7-11(12)16(20)21/h2-7,17H,8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225225
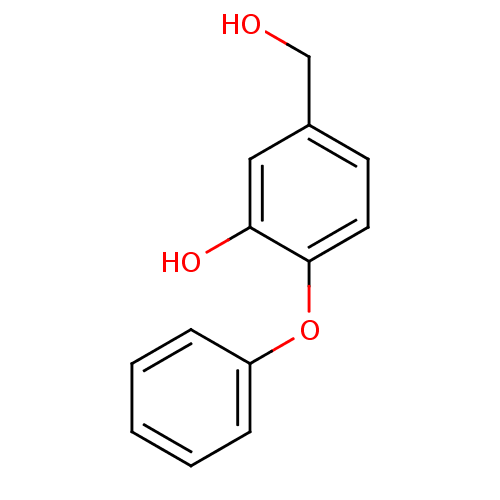
(5-(hydroxymethyl)-2-phenoxyphenol | CHEMBL238590)Show InChI InChI=1S/C13H12O3/c14-9-10-6-7-13(12(15)8-10)16-11-4-2-1-3-5-11/h1-8,14-15H,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225226
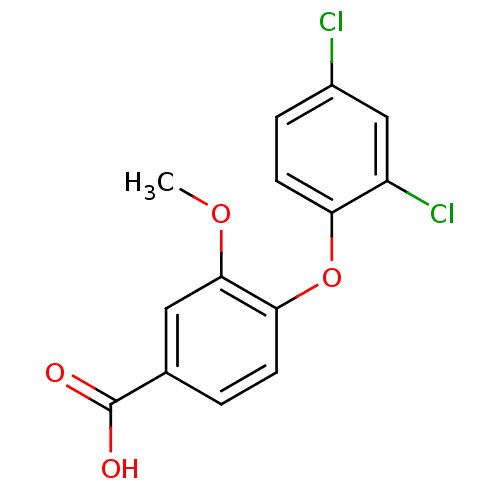
(4-(2,4-dichlorophenoxy)-3-methoxybenzoic acid | CH...)Show InChI InChI=1S/C14H10Cl2O4/c1-19-13-6-8(14(17)18)2-4-12(13)20-11-5-3-9(15)7-10(11)16/h2-7H,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 475 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225226

(4-(2,4-dichlorophenoxy)-3-methoxybenzoic acid | CH...)Show InChI InChI=1S/C14H10Cl2O4/c1-19-13-6-8(14(17)18)2-4-12(13)20-11-5-3-9(15)7-10(11)16/h2-7H,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to transthyretin at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225230

(4-(2,4-dinitrophenoxy)-3-methoxybenzoic acid | CHE...)Show SMILES COc1cc(ccc1Oc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C14H10N2O8/c1-23-13-6-8(14(17)18)2-4-12(13)24-11-5-3-9(15(19)20)7-10(11)16(21)22/h2-7H,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225228
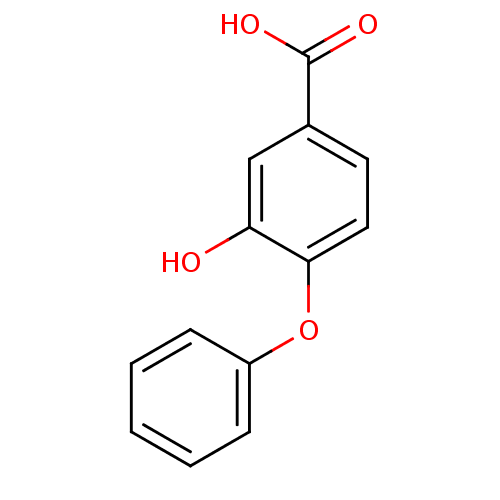
(3-hydroxy-4-phenoxybenzoic acid | CHEMBL241454)Show InChI InChI=1S/C13H10O4/c14-11-8-9(13(15)16)6-7-12(11)17-10-4-2-1-3-5-10/h1-8,14H,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225219

(3-methoxy-4-phenoxybenzaldehyde | CHEMBL241453)Show InChI InChI=1S/C14H12O3/c1-16-14-9-11(10-15)7-8-13(14)17-12-5-3-2-4-6-12/h2-10H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225224

(4-(2,4-dichlorophenoxy)-3-hydroxybenzaldehyde | CH...)Show InChI InChI=1S/C13H8Cl2O3/c14-9-2-4-12(10(15)6-9)18-13-3-1-8(7-16)5-11(13)17/h1-7,17H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transthyretin
(Homo sapiens (Human)) | BDBM50225228

(3-hydroxy-4-phenoxybenzoic acid | CHEMBL241454)Show InChI InChI=1S/C13H10O4/c14-11-8-9(13(15)16)6-7-12(11)17-10-4-2-1-3-5-10/h1-8,14H,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to transthyretin at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |
Transthyretin
(Homo sapiens (Human)) | BDBM50225218

(4-(chloromethyl)-2-methoxy-1-phenoxybenzene | CHEM...)Show InChI InChI=1S/C14H13ClO2/c1-16-14-9-11(10-15)7-8-13(14)17-12-5-3-2-4-6-12/h2-9H,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Institute of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of transthyretin fibril formation at pH 4.4 |
J Med Chem 50: 5589-99 (2007)
Article DOI: 10.1021/jm0700159
BindingDB Entry DOI: 10.7270/Q2PG1RGD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


 Purchase
Purchase




 Purchase
Purchase