 Found 11 hits in this display
Found 11 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50464142
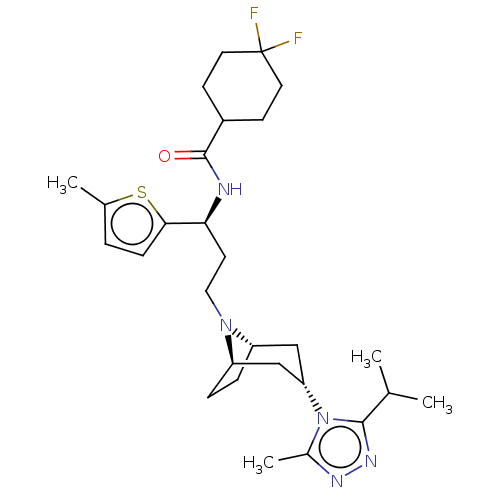
(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p... |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM313979
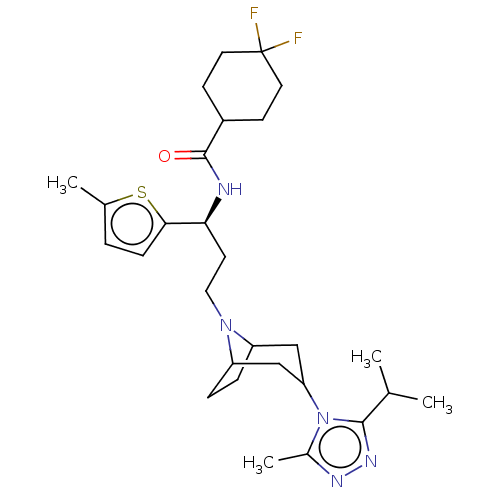
(US10167299, Example 29 | US10167299, Example 73)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,THB:8:9:16:12.13| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21?,22?,23?,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.28 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES
US Patent
| Assay Description
1. HEK293 cells which can stably express CCR5 were inoculated in a 96-well plate and incubated overnight.2. The medium in each well into which cells ... |
US Patent US10167299 (2019)
BindingDB Entry DOI: 10.7270/Q2V126X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM313979

(US10167299, Example 29 | US10167299, Example 73)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,THB:8:9:16:12.13| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21?,22?,23?,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.23 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES
US Patent
| Assay Description
1. HEK293 cells which can stably express CCR5 were inoculated in a 96-well plate and incubated overnight.2. The medium in each well into which cells ... |
US Patent US10167299 (2019)
BindingDB Entry DOI: 10.7270/Q2V126X2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells at holding potential of -80 mV after 60 secs by patch clamp assay |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM313979

(US10167299, Example 29 | US10167299, Example 73)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,THB:8:9:16:12.13| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21?,22?,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES
US Patent
| Assay Description
1. CHO-hERG cells which have been incubated overnight were added with sample buffer and incubated for 90 minutes at room temperature in darkness.2. T... |
US Patent US10167299 (2019)
BindingDB Entry DOI: 10.7270/Q2V126X2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using midazolam as substrate |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using testosterone as substrate |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50464142

(CHEMBL4248282)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(C)s1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C28H41F2N5OS/c1-17(2)26-33-32-19(4)35(26)23-15-21-6-7-22(16-23)34(21)14-11-24(25-8-5-18(3)37-25)31-27(36)20-9-12-28(29,30)13-10-20/h5,8,17,20-24H,6-7,9-16H2,1-4H3,(H,31,36)/t21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 61: 9621-9636 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01077
BindingDB Entry DOI: 10.7270/Q2W95CVF |
More data for this
Ligand-Target Pair | |