 Found 17 hits in this display
Found 17 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Macaca fascicularis) | BDBM50583787
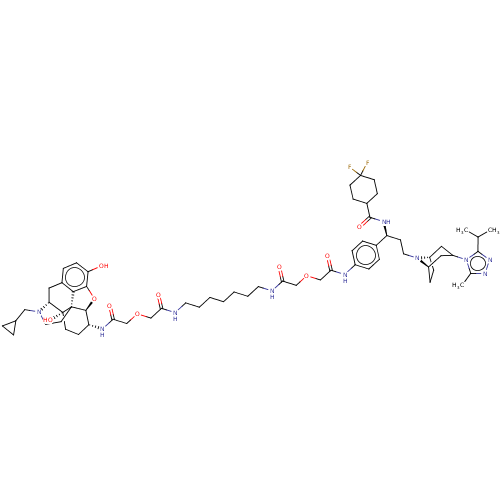
(CHEMBL5071060)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:19:18:7.6.8:2.3,83:82:70.87.69:65| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells incubated for 90 mins by competitive radioligand binding... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00408
BindingDB Entry DOI: 10.7270/Q2R49VPJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50558604
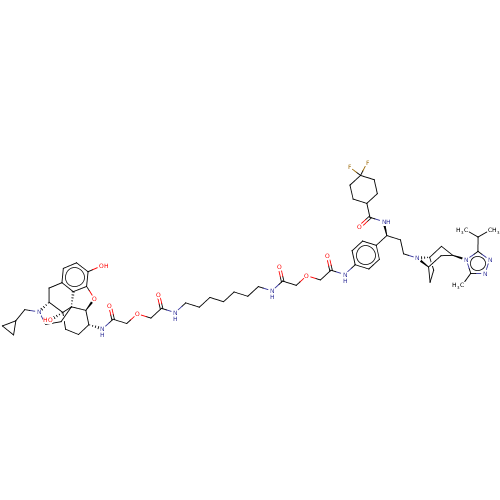
(CHEMBL4748891)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:9:7:18:3.2,83:82:65:87.70.69,19:18:6.7.8:3.2| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 51.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
US Patent
| Assay Description
A bivalent ligand 1 (FIG. 14) that combines the pharmacophores of naltrexone (a MOR antagonist) and maraviroc (a CCR5 antagonist) into one molecule w... |
US Patent US9107954 (2015)
BindingDB Entry DOI: 10.7270/Q27943D1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50583787

(CHEMBL5071060)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:19:18:7.6.8:2.3,83:82:70.87.69:65| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00408
BindingDB Entry DOI: 10.7270/Q2R49VPJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from human mu opioid receptor expressed in CHO cells after 1 hr |
Medchemcomm 4: 847-851 (2013)
Article DOI: 10.1039/c3md00080j
BindingDB Entry DOI: 10.7270/Q2474DTH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
US Patent
| Assay Description
Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... |
US Patent US9107954 (2015)
BindingDB Entry DOI: 10.7270/Q27943D1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Macaca fascicularis) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis |
Medchemcomm 4: 847-851 (2013)
Article DOI: 10.1039/c3md00080j
BindingDB Entry DOI: 10.7270/Q2474DTH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Macaca fascicularis) | BDBM50558604

(CHEMBL4748891)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:9:7:18:3.2,83:82:65:87.70.69,19:18:6.7.8:3.2| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558604

(CHEMBL4748891)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:9:7:18:3.2,83:82:65:87.70.69,19:18:6.7.8:3.2| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in Gqi5 transfected CHO cells assessed as inhibition of DAMGO-stimulated Ca2+ influx preinc... |
Medchemcomm 4: 847-851 (2013)
Article DOI: 10.1039/c3md00080j
BindingDB Entry DOI: 10.7270/Q2474DTH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
US Patent
| Assay Description
As Ca2+ flux is associated with the activation of the MOR, the functional activity of bivalent ligand 1, monovalent ligand 2, and naltrexone was then... |
US Patent US9107954 (2015)
BindingDB Entry DOI: 10.7270/Q27943D1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50583787

(CHEMBL5071060)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:19:18:7.6.8:2.3,83:82:70.87.69:65| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at mouse MOR expressed in CHO cells assessed as inhibition of DAMGO-induced calcium mobilization preincubated for 60 mins measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00408
BindingDB Entry DOI: 10.7270/Q2R49VPJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
US Patent
| Assay Description
Then the Ca2+ functional activity of bivalent ligand 1 was evaluated in the Gqi5 transfected CCR5-MOLT-4 cells as described in the literature.3 As ex... |
US Patent US9107954 (2015)
BindingDB Entry DOI: 10.7270/Q27943D1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50583787

(CHEMBL5071060)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:19:18:7.6.8:2.3,83:82:70.87.69:65| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 (unknown origin) expressed in HOS cells co-expressing Gqi-5 assessed as inhibition CCL5-stimulated Ca2+ mobilization by c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00408
BindingDB Entry DOI: 10.7270/Q2R49VPJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM160931

(US9107954, 1)Show SMILES CC(C)c1nnc(C)n1C1CC2CCC(C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:17:16:12.13:15.10.9,THB:8:9:16:12.13| Show InChI InChI=1S/C64H90F2N10O10/c1-39(2)60-73-72-40(3)76(60)48-32-46-16-17-47(33-48)75(46)29-22-49(71-61(82)43-19-23-62(65,66)24-20-43)42-11-14-45(15-12-42)69-55(80)37-84-35-53(78)67-27-7-5-4-6-8-28-68-54(79)36-85-38-56(81)70-50-21-25-64(83)52-31-44-13-18-51(77)58-57(44)63(64,59(50)86-58)26-30-74(52)34-41-9-10-41/h11-15,18,39,41,43,46-50,52,59,77,83H,4-10,16-17,19-38H2,1-3H3,(H,67,78)(H,68,79)(H,69,80)(H,70,81)(H,71,82)/t46?,47?,48?,49-,50+,52+,59-,63-,64+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in Gqi5 transfected human MOLT4 cells assessed as inhibition of RANTES-stimulated Ca2+ influx preincubated for 15 mins fo... |
Medchemcomm 4: 847-851 (2013)
Article DOI: 10.1039/c3md00080j
BindingDB Entry DOI: 10.7270/Q2474DTH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50558604

(CHEMBL4748891)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:9:7:18:3.2,83:82:65:87.70.69,19:18:6.7.8:3.2| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.059
BindingDB Entry DOI: 10.7270/Q2HD80CC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50583787

(CHEMBL5071060)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccc(NC(=O)COCC(=O)NCCCCCCCNC(=O)COCC(=O)N[C@@H]2CC[C@@]3(O)[C@@]4([H])Cc5ccc(O)c6O[C@]2([H])[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:19:18:7.6.8:2.3,83:82:70.87.69:65| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CCR5-mediated HIV-1 Bal entry in human GHOST CCR5 cells assessed as decrease in viral reverse transcriptase activity by measuring [3H]t... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00408
BindingDB Entry DOI: 10.7270/Q2R49VPJ |
More data for this
Ligand-Target Pair | |