 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM69609
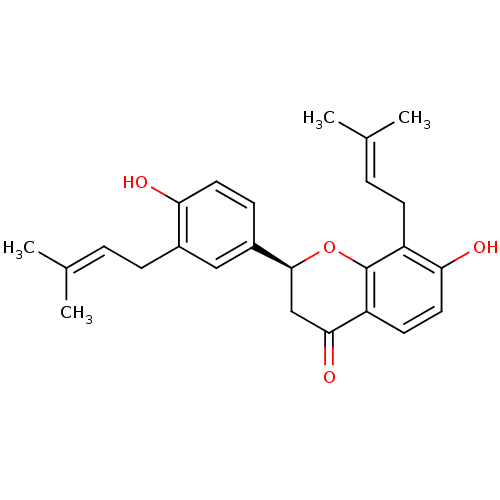
((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins |
Bioorg Med Chem 25: 3706-3713 (2017)
Article DOI: 10.1016/j.bmc.2017.05.009
BindingDB Entry DOI: 10.7270/Q2C82CRZ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay |
J Nat Prod 77: 563-70 (2014)
Article DOI: 10.1021/np400817j
BindingDB Entry DOI: 10.7270/Q23J3GZJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay |
J Nat Prod 77: 563-70 (2014)
Article DOI: 10.1021/np400817j
BindingDB Entry DOI: 10.7270/Q23J3GZJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay |
J Nat Prod 77: 563-70 (2014)
Article DOI: 10.1021/np400817j
BindingDB Entry DOI: 10.7270/Q23J3GZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins |
J Nat Prod 80: 334-346 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00783
BindingDB Entry DOI: 10.7270/Q2XD146V |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay |
J Nat Prod 77: 563-70 (2014)
Article DOI: 10.1021/np400817j
BindingDB Entry DOI: 10.7270/Q23J3GZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate |
Bioorg Med Chem Lett 23: 5836-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.102
BindingDB Entry DOI: 10.7270/Q28W3FQ0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM69609

((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]-1 Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-13-18(8-11-21(17)26)24-14-23(28)20-10-12-22(27)19(25(20)29-24)9-6-16(3)4/h5-6,8,10-13,24,26-27H,7,9,14H2,1-4H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | 5.84E+4 | n/a | n/a | n/a | n/a |
Broad Institute
Curated by PubChem BioAssay
| Assay Description
Keywords: GSK3beta, dose response, kinase, inhibition, HTS Assay Overview: The glycogen synthase kinase-3 beta (GSK-3b) is a known master regulator f... |
PubChem Bioassay (2010)
BindingDB Entry DOI: 10.7270/Q2TX3CTT |
More data for this
Ligand-Target Pair | |