 Found 12 hits in this display
Found 12 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM208769
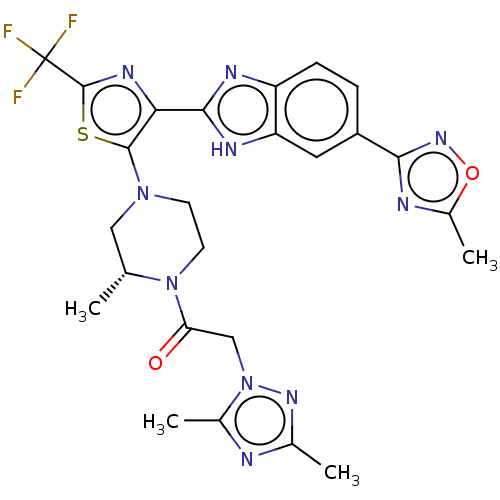
(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3/Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Actelion Pharmaceuticals Ltd.
US Patent
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
US Patent US9266876 (2016)
BindingDB Entry DOI: 10.7270/Q2DB80NB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 239 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM208769

(US9266876, 236)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1nc(C)nc1C)c1sc(nc1-c1nc2ccc(cc2[nH]1)-c1noc(C)n1)C(F)(F)F Show InChI InChI=1S/C25H25F3N10O2S/c1-12-10-36(7-8-37(12)19(39)11-38-14(3)29-13(2)34-38)23-20(33-24(41-23)25(26,27)28)22-31-17-6-5-16(9-18(17)32-22)21-30-15(4)40-35-21/h5-6,9,12H,7-8,10-11H2,1-4H3,(H,31,32)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00676
BindingDB Entry DOI: 10.7270/Q2222ZSK |
More data for this
Ligand-Target Pair | |