 Found 7 hits in this display
Found 7 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxa40
(Acinetobacter baumannii) | BDBM50140671
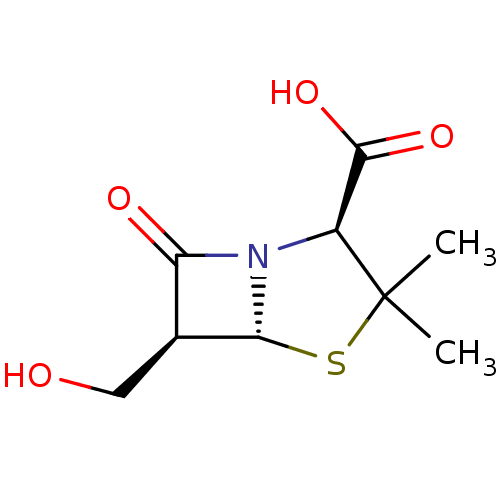
((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C(O)=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University
| Assay Description
Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. |
J Biol Chem 286: 37292-303 (2011)
Article DOI: 10.1074/jbc.M111.280115
BindingDB Entry DOI: 10.7270/Q2QV3K38 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076676

(CHEMBL177463 | Sodium; (2S,5R,6S)-6-hydroxymethyl-...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C([O-])=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/p-1/t4-,5-,7+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50140671

((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C(O)=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Beta-Lactamase inhibitory activity against representativeclass C (P99) serine enzyme |
Bioorg Med Chem Lett 14: 1299-304 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.037
BindingDB Entry DOI: 10.7270/Q22Z14ZG |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacillus cereus) | BDBM50140671

((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C(O)=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Inhibition of class B (BCII) metallo-beta-lactamase representative enzyme |
Bioorg Med Chem Lett 14: 1299-304 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.037
BindingDB Entry DOI: 10.7270/Q22Z14ZG |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50140671

((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C(O)=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Beta-Lactamase Inhibition of metallo-beta-lactamase representative class B (L1) enzyme |
Bioorg Med Chem Lett 14: 1299-304 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.037
BindingDB Entry DOI: 10.7270/Q22Z14ZG |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076676

(CHEMBL177463 | Sodium; (2S,5R,6S)-6-hydroxymethyl-...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C([O-])=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/p-1/t4-,5-,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50140671

((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...)Show SMILES CC1(C)S[C@@H]2[C@@H](CO)C(=O)N2[C@H]1C(O)=O Show InChI InChI=1S/C9H13NO4S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)15-9/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University
Curated by ChEMBL
| Assay Description
Beta-Lactamase inhibitory activity against representative class A (TEM-1) serine enzyme |
Bioorg Med Chem Lett 14: 1299-304 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.037
BindingDB Entry DOI: 10.7270/Q22Z14ZG |
More data for this
Ligand-Target Pair | |