 Found 5 hits in this display
Found 5 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50089197
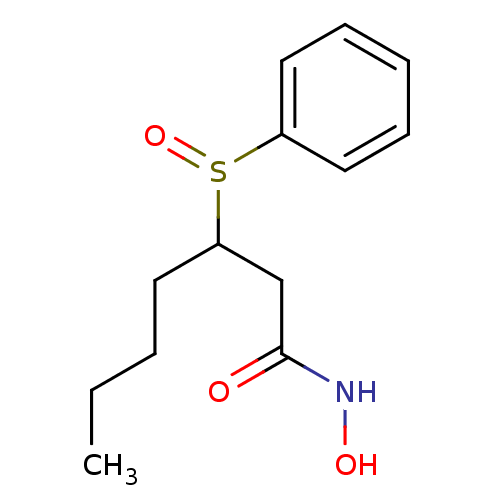
(3-Benzenesulfinyl-heptanoic acid hydroxyamide | CH...)Show InChI InChI=1S/C13H19NO3S/c1-2-3-7-12(10-13(15)14-16)18(17)11-8-5-4-6-9-11/h4-6,8-9,12,16H,2-3,7,10H2,1H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-1 |
J Med Chem 43: 2324-31 (2000)
BindingDB Entry DOI: 10.7270/Q2QF8TJR |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50089197

(3-Benzenesulfinyl-heptanoic acid hydroxyamide | CH...)Show InChI InChI=1S/C13H19NO3S/c1-2-3-7-12(10-13(15)14-16)18(17)11-8-5-4-6-9-11/h4-6,8-9,12,16H,2-3,7,10H2,1H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. |
J Med Chem 43: 2324-31 (2000)
BindingDB Entry DOI: 10.7270/Q2QF8TJR |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50089197

(3-Benzenesulfinyl-heptanoic acid hydroxyamide | CH...)Show InChI InChI=1S/C13H19NO3S/c1-2-3-7-12(10-13(15)14-16)18(17)11-8-5-4-6-9-11/h4-6,8-9,12,16H,2-3,7,10H2,1H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neutral endopeptidase enzyme (NEP). |
J Med Chem 43: 2324-31 (2000)
BindingDB Entry DOI: 10.7270/Q2QF8TJR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50089197

(3-Benzenesulfinyl-heptanoic acid hydroxyamide | CH...)Show InChI InChI=1S/C13H19NO3S/c1-2-3-7-12(10-13(15)14-16)18(17)11-8-5-4-6-9-11/h4-6,8-9,12,16H,2-3,7,10H2,1H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme (ACE) |
J Med Chem 43: 2324-31 (2000)
BindingDB Entry DOI: 10.7270/Q2QF8TJR |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM50089197

(3-Benzenesulfinyl-heptanoic acid hydroxyamide | CH...)Show InChI InChI=1S/C13H19NO3S/c1-2-3-7-12(10-13(15)14-16)18(17)11-8-5-4-6-9-11/h4-6,8-9,12,16H,2-3,7,10H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Endothelin-converting enzyme 1 |
J Med Chem 43: 2324-31 (2000)
BindingDB Entry DOI: 10.7270/Q2QF8TJR |
More data for this
Ligand-Target Pair | |