 Found 8 hits in this display
Found 8 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282210
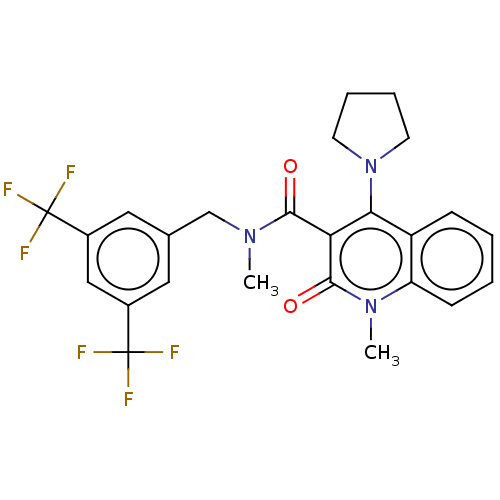
(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SMS2 expressed in HEK293T cells using C2-ceramide as substrate preincubated for 60 mins followed by substrate addition measured a... |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of thymidylate synthase obtained from human |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SMS2 |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal FLAG-tagged SMS2 expressed in Freestyle293 cell membrane using C14-phosphatidylcholineD72 and C17-ceramide... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115376
BindingDB Entry DOI: 10.7270/Q2J106QT |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Mus musculus) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant C-terminal FLAG-tagged SMS2 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115376
BindingDB Entry DOI: 10.7270/Q2J106QT |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of glycinamide ribonucleotide transformylase obtained from porcine (GAR Tfase) |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SMS1 |
Eur J Med Chem 136: 283-293 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.067
BindingDB Entry DOI: 10.7270/Q29G5QB9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50282210

(CHEMBL4162182)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1c(N2CCCC2)c2ccccc2n(C)c1=O Show InChI InChI=1S/C25H23F6N3O2/c1-32(14-15-11-16(24(26,27)28)13-17(12-15)25(29,30)31)22(35)20-21(34-9-5-6-10-34)18-7-3-4-8-19(18)33(2)23(20)36/h3-4,7-8,11-13H,5-6,9-10,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal FLAG-tagged SMS1 expressed in mammalian expression system using C14-phosphatidylcholineD72 and C17-ceramid... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115376
BindingDB Entry DOI: 10.7270/Q2J106QT |
More data for this
Ligand-Target Pair | |