 Found 7 hits in this display
Found 7 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM199356
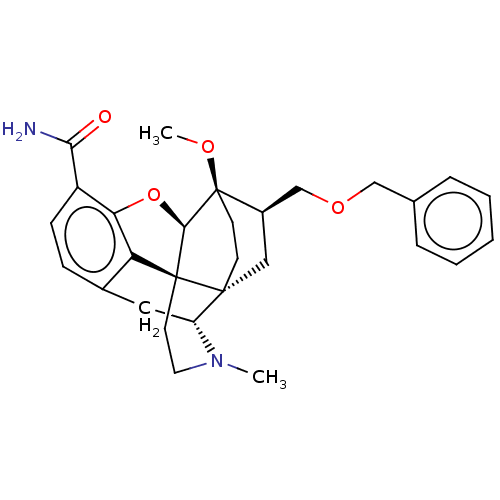
(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 17.3 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand dose-displacement binding assays for t-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 185 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.
US Patent
| Assay Description
Delta-opioid Receptor Binding Assay Procedures were conducted as
follows. Radioligand dose-displacement assays used 0.3 nM
[3H]-Naltrindole (Perkin... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Opioid growth factor receptor-like protein 1
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 8.62E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P.
US Patent
| Assay Description
Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (Perkin Elmer, Shelton, Conn.; 87.7 Ci/mmole) with 12 ug mem... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 86 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.
US Patent
| Assay Description
Functional [35S]GTPgammaS binding assays were conducted as follows.
Delta opioid receptor membrane solution was prepared by sequentially
adding fin... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 102 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.
US Patent
| Assay Description
[35S]GTPgammaS functional assays were conducted using freshly thawed u-receptor membranes (Perkin Elmer, Shelton, Conn.). Assay reactions
were prepa... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM199356

(US9221831, 21)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1COCc1ccccc1)[C@H]1Cc4ccc(C(N)=O)c5O[C@@H]2[C@]3(CCN1C)c45 |r,TLB:8:7:29.28:4.3| Show InChI InChI=1S/C29H34N2O4/c1-31-13-12-28-23-19-8-9-21(25(30)32)24(23)35-26(28)29(33-2)11-10-27(28,22(31)14-19)15-20(29)17-34-16-18-6-4-3-5-7-18/h3-9,20,22,26H,10-17H2,1-2H3,(H2,30,32)/t20-,22-,26-,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 13.3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.
US Patent
| Assay Description
Functional [35S]GTPgammaS binding assays were conducted as follows. κ
opioid receptor membrane solution was prepared by sequentially adding
final ... |
US Patent US9221831 (2015)
BindingDB Entry DOI: 10.7270/Q2T72G8M |
More data for this
Ligand-Target Pair | |