 Found 4 hits in this display
Found 4 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM136059
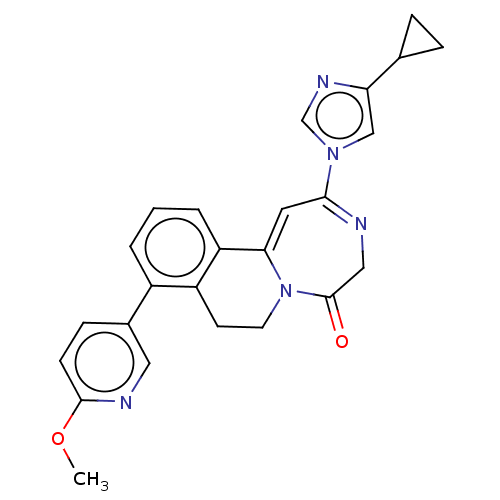
(US8853203, 99d | US9650377, Example 99d)Show SMILES COc1ccc(cn1)-c1cccc2C3=CC(=NCC(=O)N3CCc12)n1cnc(c1)C1CC1 |c:16,t:14| Show InChI InChI=1S/C25H23N5O2/c1-32-24-8-7-17(12-27-24)18-3-2-4-20-19(18)9-10-30-22(20)11-23(26-13-25(30)31)29-14-21(28-15-29)16-5-6-16/h2-4,7-8,11-12,14-16H,5-6,9-10,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... |
US Patent US9650377 (2017)
BindingDB Entry DOI: 10.7270/Q2G73GT2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM136059

(US8853203, 99d | US9650377, Example 99d)Show SMILES COc1ccc(cn1)-c1cccc2C3=CC(=NCC(=O)N3CCc12)n1cnc(c1)C1CC1 |c:16,t:14| Show InChI InChI=1S/C25H23N5O2/c1-32-24-8-7-17(12-27-24)18-3-2-4-20-19(18)9-10-30-22(20)11-23(26-13-25(30)31)29-14-21(28-15-29)16-5-6-16/h2-4,7-8,11-12,14-16H,5-6,9-10,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... |
US Patent US8853203 (2014)
BindingDB Entry DOI: 10.7270/Q2MP520T |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM136059

(US8853203, 99d | US9650377, Example 99d)Show SMILES COc1ccc(cn1)-c1cccc2C3=CC(=NCC(=O)N3CCc12)n1cnc(c1)C1CC1 |c:16,t:14| Show InChI InChI=1S/C25H23N5O2/c1-32-24-8-7-17(12-27-24)18-3-2-4-20-19(18)9-10-30-22(20)11-23(26-13-25(30)31)29-14-21(28-15-29)16-5-6-16/h2-4,7-8,11-12,14-16H,5-6,9-10,13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... |
US Patent US9650377 (2017)
BindingDB Entry DOI: 10.7270/Q2G73GT2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM136059

(US8853203, 99d | US9650377, Example 99d)Show SMILES COc1ccc(cn1)-c1cccc2C3=CC(=NCC(=O)N3CCc12)n1cnc(c1)C1CC1 |c:16,t:14| Show InChI InChI=1S/C25H23N5O2/c1-32-24-8-7-17(12-27-24)18-3-2-4-20-19(18)9-10-30-22(20)11-23(26-13-25(30)31)29-14-21(28-15-29)16-5-6-16/h2-4,7-8,11-12,14-16H,5-6,9-10,13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... |
US Patent US8853203 (2014)
BindingDB Entry DOI: 10.7270/Q2MP520T |
More data for this
Ligand-Target Pair | |