 Found 9 hits in this display
Found 9 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50359629
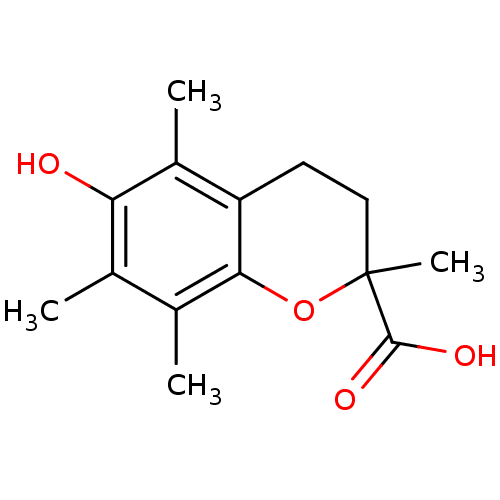
(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Product Research and Infection Biology
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 17: 2558-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.008
BindingDB Entry DOI: 10.7270/Q2HD7WHJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Product Research and Infection Biology
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 17: 2558-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.008
BindingDB Entry DOI: 10.7270/Q2HD7WHJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Anti-oxidant activity in DPPH radical scavenging assay; n=3-4 |
Bioorg Med Chem Lett 15: 1793-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.039
BindingDB Entry DOI: 10.7270/Q20V8DJD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Skin Biotechnology Center
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 mins |
Bioorg Med Chem Lett 21: 7466-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.122
BindingDB Entry DOI: 10.7270/Q2DR2VZK |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Product Research and Infection Biology
Curated by ChEMBL
| Assay Description
Inhibition of Xanthine oxidase |
Bioorg Med Chem Lett 17: 2558-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.008
BindingDB Entry DOI: 10.7270/Q2HD7WHJ |
More data for this
Ligand-Target Pair | |
Dehydrogenase/reductase SDR family member 9
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Product Research and Infection Biology
Curated by ChEMBL
| Assay Description
Inhibition of 3alphaHSD |
Bioorg Med Chem Lett 17: 2558-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.008
BindingDB Entry DOI: 10.7270/Q2HD7WHJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50359629

(TROLOX)Show InChI InChI=1S/C14H18O4/c1-7-8(2)12-10(9(3)11(7)15)5-6-14(4,18-12)13(16)17/h15H,5-6H2,1-4H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Instituto Superior Técnico
| Assay Description
AChE enzymatic activity was measured using an adaptation of the method previously described [Ingkaninan et al., J. Ethnopharmacol., 89:261-264]; 98 &... |
J Enzyme Inhib Med Chem 26: 485-97 (2011)
Article DOI: 10.3109/14756366.2010.529806
BindingDB Entry DOI: 10.7270/Q2862FBS |
More data for this
Ligand-Target Pair | |