 Found 6 hits in this display
Found 6 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019293
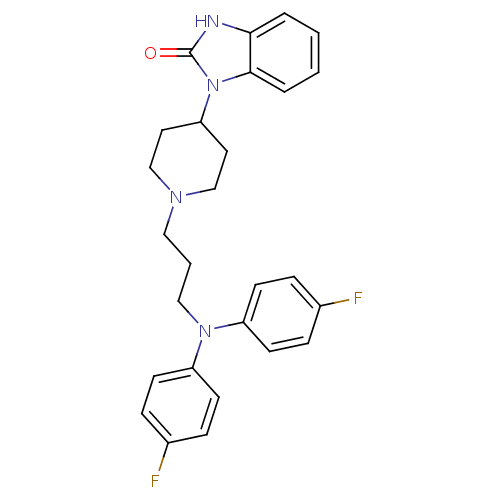
(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
X-box-binding protein 1
(Homo sapiens (Human)) | BDBM50019293

(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PCBioAssay
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
| Assay Description
NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Dr. Randal Kaufman, Univer... |
PubChem Bioassay (2011)
BindingDB Entry DOI: 10.7270/Q2NK3CGB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Rattus norvegicus) | BDBM50019293

(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against type IIA sodium channel in CNaIIA-1 cell line expressed in CHO cells |
J Med Chem 39: 1514-20 (1996)
Article DOI: 10.1021/jm950467y
BindingDB Entry DOI: 10.7270/Q2JS9PJC |
More data for this
Ligand-Target Pair | |
DNA damage-inducible transcript 3 protein
(Mus musculus) | BDBM50019293

(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PCBioAssay
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
| Assay Description
NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Dr. Randal Kaufman, Univer... |
PubChem Bioassay (2010)
BindingDB Entry DOI: 10.7270/Q2PC30VZ |
More data for this
Ligand-Target Pair | |
DNA damage-inducible transcript 3 protein
(Mus musculus) | BDBM50019293

(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PCBioAssay
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
| Assay Description
NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Dr. Randal Kaufman, Univer... |
PubChem Bioassay (2011)
BindingDB Entry DOI: 10.7270/Q28C9TQN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50019293

(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 receptor |
Bioorg Med Chem 18: 5938-44 (2010)
Article DOI: 10.1016/j.bmc.2010.06.082
BindingDB Entry DOI: 10.7270/Q2ZS2WQ5 |
More data for this
Ligand-Target Pair | |