 Found 17 hits in this display
Found 17 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50466671
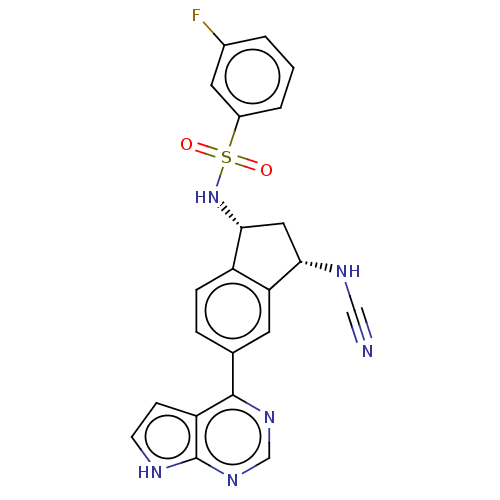
(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged JAK3 JH1 domain using KAIETDKEYYTVKD-NH2 as substrate in presence of 1 mM ATP concentration by coupled PK/... |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 in human PBMC assessed as reduction in IL15-induced STAT5 phosphorylation preincubated for 75 mins followed by IL15 addition and m... |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged JAK1 using KAIETDKEYYTVKD-NH2 as substrate in presence of 1 mM ATP concentration by coupled PK/LDH assay |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged JAK2 using KAIETDKEYYTVKD-NH2 as substrate in presence of 1 mM ATP concentration by coupled PK/LDH assay |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BMX (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged TYK2 expressed in SF21/baculovirus expression system using KAIETDKEYYTVKD-NH2 as substrate in presence of ... |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TXK (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1/TYK2 in human PBMC assessed as reduction in IL10-induced STAT3 phosphorylation preincubated for 75 mins followed by IL10 addition ... |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 7
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAP2K7 (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BLK (unknown origin) in presence of 1 mM ATP concentration |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50466671

(CHEMBL4277610)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H]1C[C@H](NC#N)c2cc(ccc12)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H17FN6O2S/c23-14-2-1-3-15(9-14)32(30,31)29-20-10-19(26-11-24)18-8-13(4-5-16(18)20)21-17-6-7-25-22(17)28-12-27-21/h1-9,12,19-20,26,29H,10H2,(H,25,27,28)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 in human whole blood assessed as reduction in IL15-induced STAT5 phosphorylation preincubated for 75 mins followed by IL15 additio... |
J Med Chem 61: 10665-10699 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01308
BindingDB Entry DOI: 10.7270/Q25T3P53 |
More data for this
Ligand-Target Pair | |