 Found 10 hits in this display
Found 10 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine protease 1
(Bos taurus (bovine)) | BDBM14332
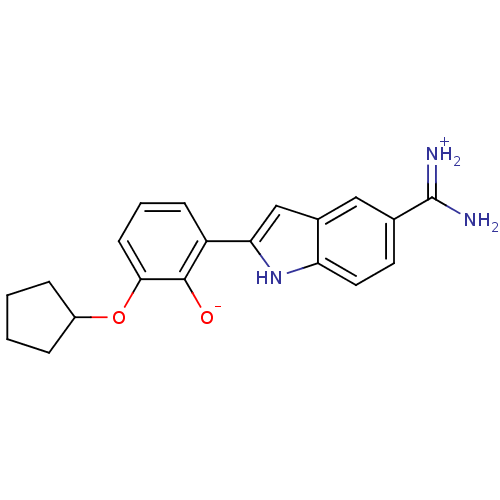
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 68 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.06 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14332

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115856
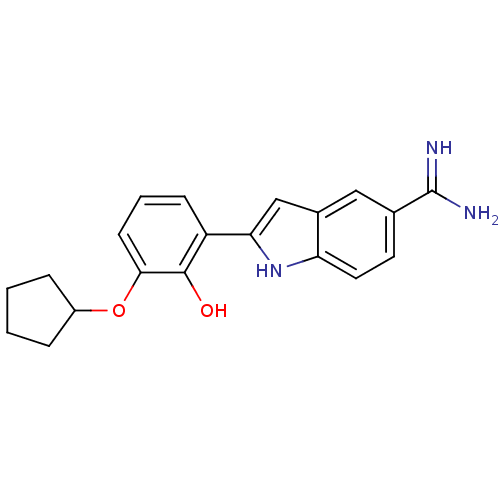
(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50115856

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115856

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of tissue-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM14332

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50115856

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14332

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50115856

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14332

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |