 Found 3 hits in this display
Found 3 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 1
(RAT) | BDBM50231744
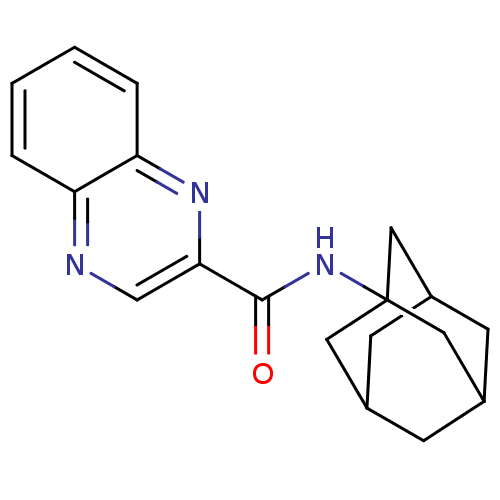
(CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1 |TLB:2:3:6:10.8.9,THB:8:7:4:10.9.11,8:9:6.7.12:4,11:9:6:12.3.4,11:3:6:10.8.9| Show InChI InChI=1S/C19H21N3O/c23-18(17-11-20-15-3-1-2-4-16(15)21-17)22-19-8-12-5-13(9-19)7-14(6-12)10-19/h1-4,11-14H,5-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50231744

(CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1 |TLB:2:3:6:10.8.9,THB:8:7:4:10.9.11,8:9:6.7.12:4,11:9:6:12.3.4,11:3:6:10.8.9| Show InChI InChI=1S/C19H21N3O/c23-18(17-11-20-15-3-1-2-4-16(15)21-17)22-19-8-12-5-13(9-19)7-14(6-12)10-19/h1-4,11-14H,5-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
| |
Mol Pharmacol 63: 1082-93 (2003)
Article DOI: 10.1124/mol.63.5.1082
BindingDB Entry DOI: 10.7270/Q2BV7F62 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50231744

(CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1 |TLB:2:3:6:10.8.9,THB:8:7:4:10.9.11,8:9:6.7.12:4,11:9:6:12.3.4,11:3:6:10.8.9| Show InChI InChI=1S/C19H21N3O/c23-18(17-11-20-15-3-1-2-4-16(15)21-17)22-19-8-12-5-13(9-19)7-14(6-12)10-19/h1-4,11-14H,5-10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 expressed in cerebellar granule cells assessed as accumulation of [3H]inositol phosphate |
J Med Chem 51: 634-47 (2008)
Article DOI: 10.1021/jm0611298
BindingDB Entry DOI: 10.7270/Q2QC04B2 |
More data for this
Ligand-Target Pair | |