 Found 5 hits in this display
Found 5 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50085531
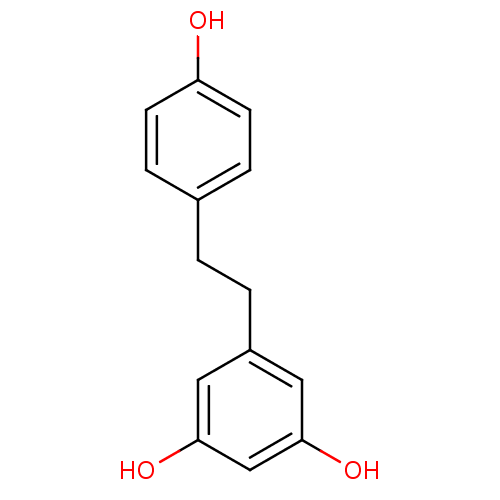
(3,4',5-trihydroxybibenzyl | 5-(4-hydroxyphenethyl)...)Show InChI InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hyogo University of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human TRPA1 expressed in HEK293 cells assessed as decrease in AITC-induced calcium influx preincubated for 6 mins followed by AITC addi... |
Bioorg Med Chem Lett 27: 3167-3172 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.025
BindingDB Entry DOI: 10.7270/Q2TH8Q4R |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50085531

(3,4',5-trihydroxybibenzyl | 5-(4-hydroxyphenethyl)...)Show InChI InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase using dibenzylfluorescein as substrate preincubated for 30 mins measured after 2 hrs by fluorimetry |
Bioorg Med Chem 20: 510-20 (2011)
Article DOI: 10.1016/j.bmc.2011.09.031
BindingDB Entry DOI: 10.7270/Q2X067GF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085531

(3,4',5-trihydroxybibenzyl | 5-(4-hydroxyphenethyl)...)Show InChI InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE |
J Med Chem 52: 4694-715 (2009)
Article DOI: 10.1021/jm900259h
BindingDB Entry DOI: 10.7270/Q2F47P6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085531

(3,4',5-trihydroxybibenzyl | 5-(4-hydroxyphenethyl)...)Show InChI InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... |
J Med Chem 52: 4694-715 (2009)
Article DOI: 10.1021/jm900259h
BindingDB Entry DOI: 10.7270/Q2F47P6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM50085531

(3,4',5-trihydroxybibenzyl | 5-(4-hydroxyphenethyl)...)Show InChI InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Hyogo University of Health Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at human TRPA1 expressed in HEK293 cells assessed as induction of calcium influx at 30 uM after 6 mins by Fluo-4 dye-based assay |
Bioorg Med Chem Lett 27: 3167-3172 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.025
BindingDB Entry DOI: 10.7270/Q2TH8Q4R |
More data for this
Ligand-Target Pair | |