 Found 23 hits Enz. Inhib. hit(s) with Target = 'Hyaluronidase-1'
Found 23 hits Enz. Inhib. hit(s) with Target = 'Hyaluronidase-1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81868

(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(CNC(=O)c2cn(Cc3ccc(F)cc3)c3ccccc23)cc1 Show InChI InChI=1S/C23H18F2N2O/c24-18-9-5-16(6-10-18)13-26-23(28)21-15-27(22-4-2-1-3-20(21)22)14-17-7-11-19(25)12-8-17/h1-12,15H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81862
(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19FN2O/c24-20-12-10-17(11-13-20)15-25-23(27)22-14-19-8-4-5-9-21(19)26(22)16-18-6-2-1-3-7-18/h1-14H,15-16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81865
(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(CNC(=O)c2cc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H18F2N2O/c24-19-9-5-16(6-10-19)14-26-23(28)22-13-18-3-1-2-4-21(18)27(22)15-17-7-11-20(25)12-8-17/h1-13H,14-15H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81866

(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19FN2O/c24-19-12-10-18(11-13-19)15-26-16-21(20-8-4-5-9-22(20)26)23(27)25-14-17-6-2-1-3-7-17/h1-13,16H,14-15H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81864

(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(Cn2c(cc3ccccc23)C(=O)NCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O/c24-19-9-5-16(6-10-19)14-26-23(28)22-13-18-3-1-2-4-21(18)27(22)15-17-7-11-20(25)12-8-17/h1-13H,14-15H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81861
(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19ClN2O/c24-20-12-10-17(11-13-20)15-25-23(27)22-14-19-8-4-5-9-21(19)26(22)16-18-6-2-1-3-7-18/h1-14H,15-16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81867
(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(Cn2cc(C(=O)NCc3ccc(Cl)cc3)c3ccccc23)cc1 Show InChI InChI=1S/C23H18ClFN2O/c24-18-9-5-16(6-10-18)13-26-23(28)21-15-27(22-4-2-1-3-20(21)22)14-17-7-11-19(25)12-8-17/h1-12,15H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81860
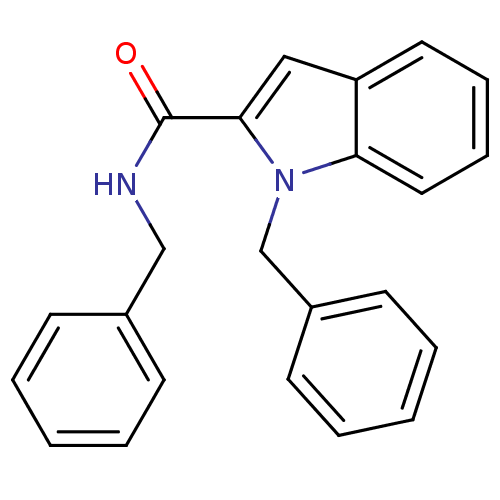
(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H20N2O/c26-23(24-16-18-9-3-1-4-10-18)22-15-20-13-7-8-14-21(20)25(22)17-19-11-5-2-6-12-19/h1-15H,16-17H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.03E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81863
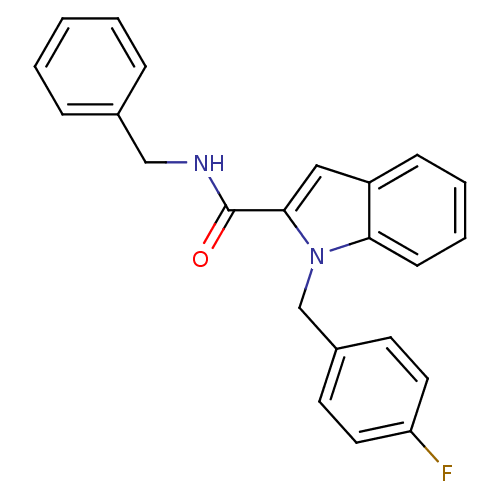
(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19FN2O/c24-20-12-10-18(11-13-20)16-26-21-9-5-4-8-19(21)14-22(26)23(27)25-15-17-6-2-1-3-7-17/h1-14H,15-16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
TBA
| Assay Description
Stains-all assay were used to test inhibitors at pH of 7. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81868

(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(CNC(=O)c2cn(Cc3ccc(F)cc3)c3ccccc23)cc1 Show InChI InChI=1S/C23H18F2N2O/c24-18-9-5-16(6-10-18)13-26-23(28)21-15-27(22-4-2-1-3-20(21)22)14-17-7-11-19(25)12-8-17/h1-12,15H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | 3.5 | 37 |
TBA
| Assay Description
Morgan-elson assay were used to test inhibitors at pH of 3.5. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81866

(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19FN2O/c24-19-12-10-18(11-13-19)15-26-16-21(20-8-4-5-9-22(20)26)23(27)25-14-17-6-2-1-3-7-17/h1-13,16H,14-15H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | 3.5 | 37 |
TBA
| Assay Description
Morgan-elson assay were used to test inhibitors at pH of 3.5. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81861

(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H19ClN2O/c24-20-12-10-17(11-13-20)15-25-23(27)22-14-19-8-4-5-9-21(19)26(22)16-18-6-2-1-3-7-18/h1-14H,15-16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | 3.5 | 37 |
TBA
| Assay Description
Morgan-elson assay were used to test inhibitors at pH of 3.5. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81867

(N-Substituted indole-2- and 3-carboxamide derivati...)Show SMILES Fc1ccc(Cn2cc(C(=O)NCc3ccc(Cl)cc3)c3ccccc23)cc1 Show InChI InChI=1S/C23H18ClFN2O/c24-18-9-5-16(6-10-18)13-26-23(28)21-15-27(22-4-2-1-3-20(21)22)14-17-7-11-19(25)12-8-17/h1-12,15H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.29E+4 | n/a | n/a | n/a | n/a | 3.5 | 37 |
TBA
| Assay Description
Morgan-elson assay were used to test inhibitors at pH of 3.5. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM81860

(N-Substituted indole-2- and 3-carboxamide derivati...)Show InChI InChI=1S/C23H20N2O/c26-23(24-16-18-9-3-1-4-10-18)22-15-20-13-7-8-14-21(20)25(22)17-19-11-5-2-6-12-19/h1-15H,16-17H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.82E+4 | n/a | n/a | n/a | n/a | 3.5 | 37 |
TBA
| Assay Description
Morgan-elson assay were used to test inhibitors at pH of 3.5. |
Chem Biol Drug Des 70: 547-51 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00590.x
BindingDB Entry DOI: 10.7270/Q2FB51F7 |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50316415
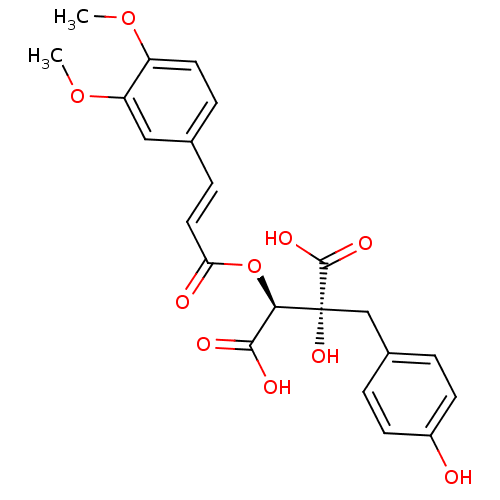
(CHEMBL1097937 | Cimicifugic acid L | cimicifugic a...)Show SMILES COc1ccc(\C=C\C(=O)O[C@H](C(O)=O)[C@](O)(Cc2ccc(O)cc2)C(O)=O)cc1OC |r| Show InChI InChI=1S/C22H22O10/c1-30-16-9-5-13(11-17(16)31-2)6-10-18(24)32-19(20(25)26)22(29,21(27)28)12-14-3-7-15(23)8-4-14/h3-11,19,23,29H,12H2,1-2H3,(H,25,26)(H,27,28)/b10-6+/t19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50316417

(CHEMBL1094303 | Cimicifugic acid N)Show SMILES COc1ccc(\C=C/C(=O)O[C@H](C(O)=O)[C@](O)(Cc2ccc(O)c(O)c2)C(O)=O)cc1OC |r| Show InChI InChI=1S/C22H22O11/c1-31-16-7-4-12(10-17(16)32-2)5-8-18(25)33-19(20(26)27)22(30,21(28)29)11-13-3-6-14(23)15(24)9-13/h3-10,19,23-24,30H,11H2,1-2H3,(H,26,27)(H,28,29)/b8-5-/t19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50316416
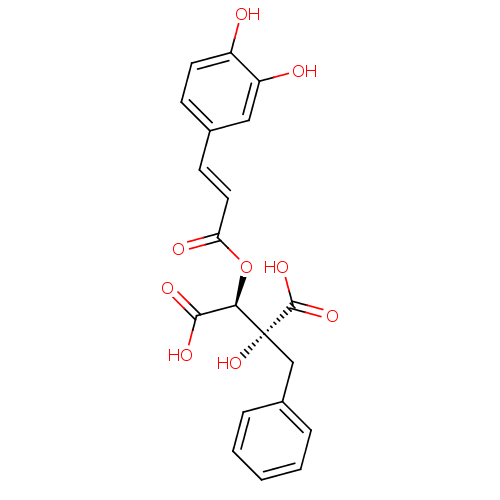
(CHEMBL1096937 | Cimicifugic acid M)Show SMILES OC(=O)[C@@H](OC(=O)\C=C\c1ccc(O)c(O)c1)[C@](O)(Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C20H18O9/c21-14-8-6-12(10-15(14)22)7-9-16(23)29-17(18(24)25)20(28,19(26)27)11-13-4-2-1-3-5-13/h1-10,17,21-22,28H,11H2,(H,24,25)(H,26,27)/b9-7+/t17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50316414
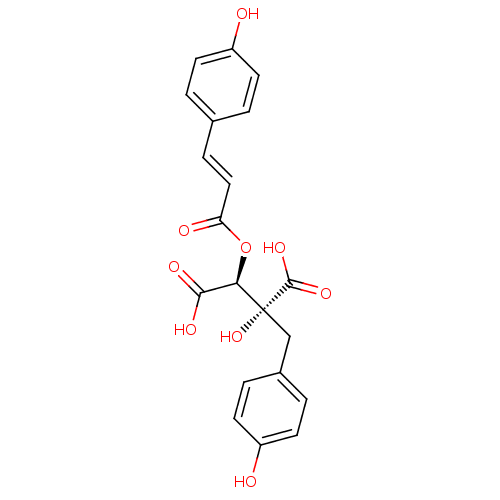
(CHEMBL1096631 | Cimicifugic acid K)Show SMILES OC(=O)[C@@H](OC(=O)\C=C\c1ccc(O)cc1)[C@](O)(Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C20H18O9/c21-14-6-1-12(2-7-14)5-10-16(23)29-17(18(24)25)20(28,19(26)27)11-13-3-8-15(22)9-4-13/h1-10,17,21-22,28H,11H2,(H,24,25)(H,26,27)/b10-5+/t17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1/Hyaluronidase-2
(Bos taurus ) | BDBM50292241

(CHEMBL4159080)Show SMILES Cl.Clc1cc(cnc1Cl)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C22H22Cl2N4O/c23-17-12-14(13-27-21(17)24)22(29)26-11-5-10-25-20-15-6-1-3-8-18(15)28-19-9-4-2-7-16(19)20/h1,3,6,8,12-13H,2,4-5,7,9-11H2,(H,25,28)(H,26,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz
Curated by ChEMBL
| Assay Description
Inhibition of bovine testes hyaluronidase using hyaluronic acid as substrate preincubated for 10 mins in dark followed by incubation with substrate f... |
Eur J Med Chem 145: 760-769 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.014
BindingDB Entry DOI: 10.7270/Q2WM1GZM |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50133496

((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...)Show SMILES OC(=O)[C@@H](Cc1ccc(O)c(O)c1)OC(=O)\C=C\c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C18H16O8/c19-12-4-1-10(7-14(12)21)3-6-17(23)26-16(18(24)25)9-11-2-5-13(20)15(22)8-11/h1-8,16,19-22H,9H2,(H,24,25)/b6-3+/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM4375
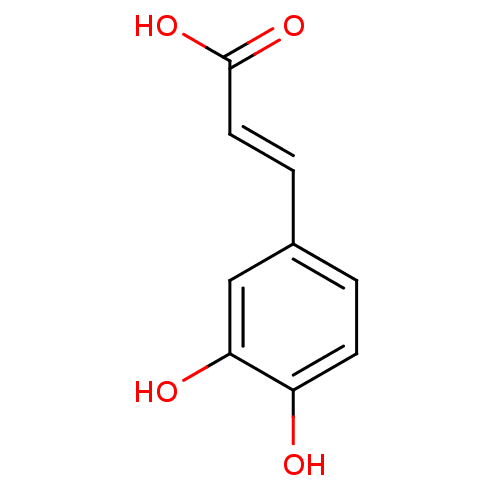
((2E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | (2...)Show InChI InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50214744

((2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic aci...)Show InChI InChI=1S/C10H10O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |
Hyaluronidase-1
(Homo sapiens (Human)) | BDBM50241245
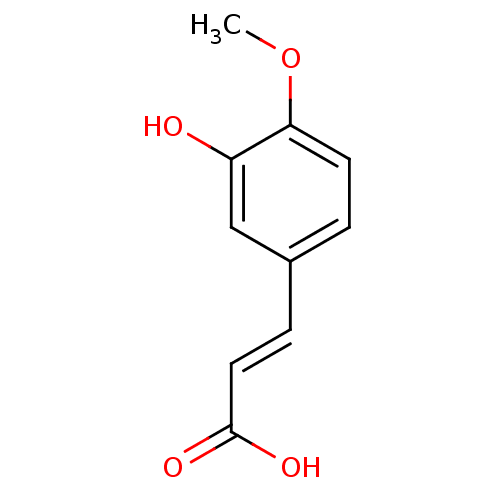
((2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoic aci...)Show InChI InChI=1S/C10H10O4/c1-14-9-4-2-7(6-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Inhibition of hyaluronidase |
J Nat Prod 73: 609-12 (2010)
Article DOI: 10.1021/np900752t
BindingDB Entry DOI: 10.7270/Q2GF0TNW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data