

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM50122619 (CHEMBL3623107) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Transrepression activity of LXR in human THP1 cells assessed as inhibition of LPS-induced IL-6 level after 18 hrs by ELISA | ACS Med Chem Lett 6: 902-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00170 BindingDB Entry DOI: 10.7270/Q2VM4F2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM50396601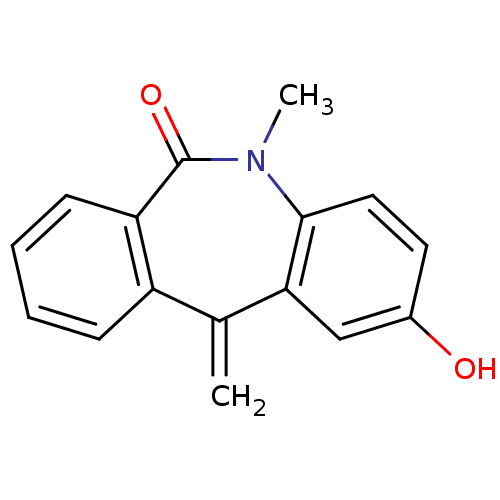 (CHEMBL2171902) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Transrepression activity of LXR in human THP1 cells assessed as inhibition of LPS-induced IL-6 level after 18 hrs by ELISA | ACS Med Chem Lett 6: 902-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00170 BindingDB Entry DOI: 10.7270/Q2VM4F2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Transrepression activity of LXR in human THP1 cells assessed as inhibition of LPS-induced IL-6 level after 18 hrs by ELISA | ACS Med Chem Lett 6: 902-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00170 BindingDB Entry DOI: 10.7270/Q2VM4F2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Agonist activity at human LXR by transactivation assay | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at LXR in human THP1 cells assessed as upregulation of ABCA1 gene expression after 24 hrs by RT-PCR analysis | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha/LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at LXR in human HepG2 cells assessed as upregulation of SREBP1C gene expression after 24 hrs by RT-PCR analysis | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||