

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526424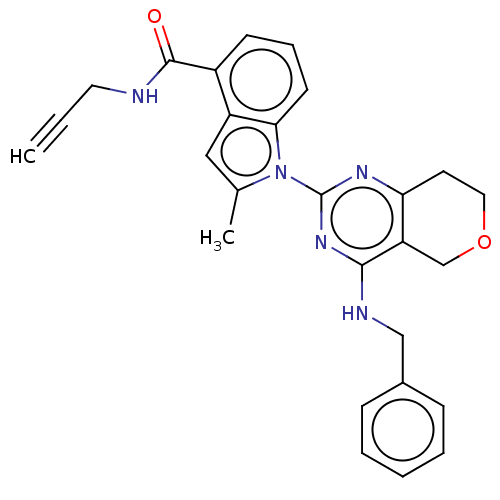 (CHEMBL4555451) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526420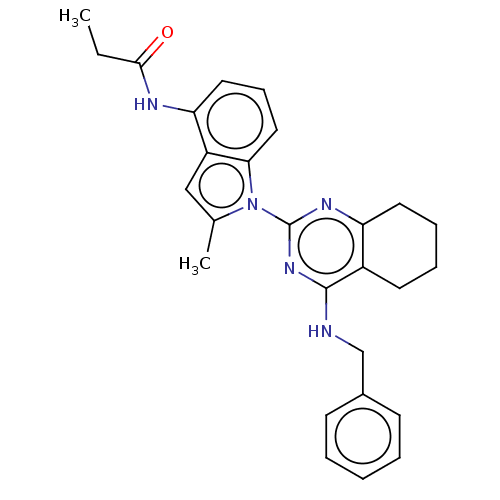 (CHEMBL4553677) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526416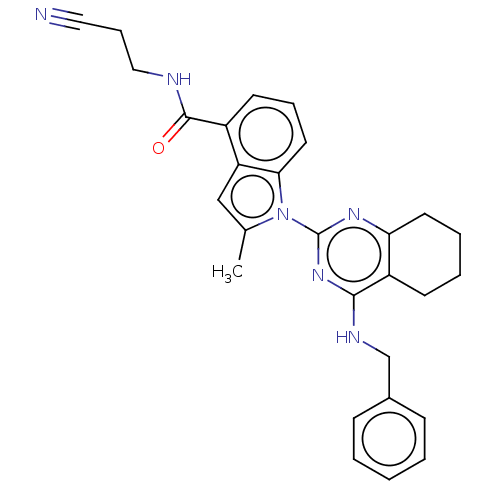 (CHEMBL4535337) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526425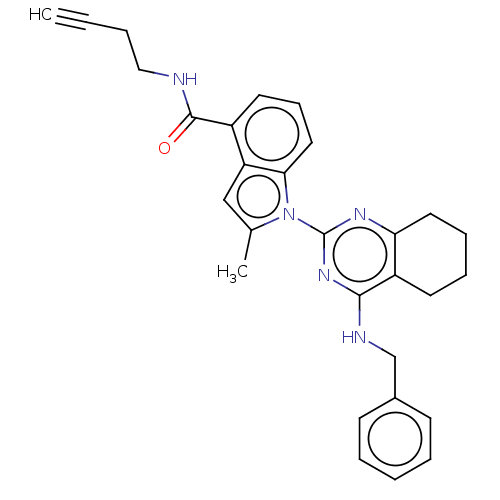 (CHEMBL4441270) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500325 (Cb 5083 | Cb-5083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50508305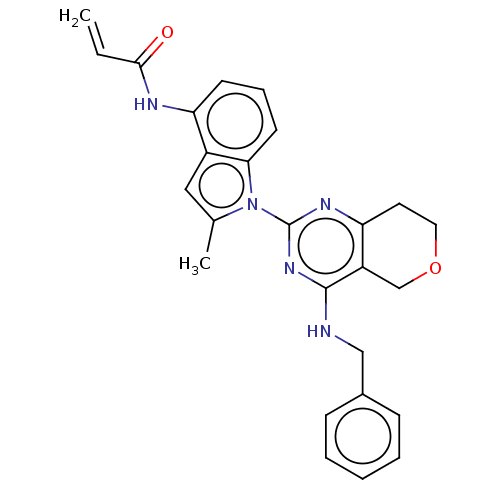 (CHEMBL4453010) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526412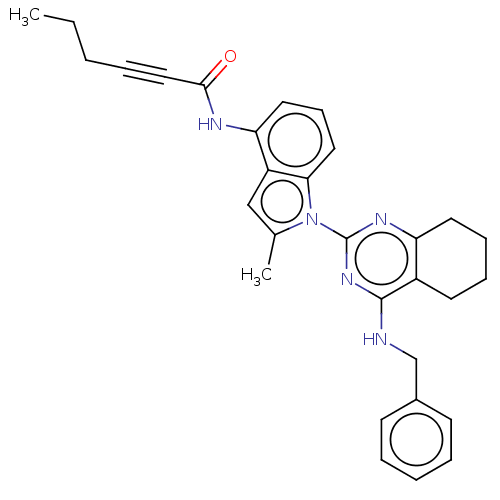 (CHEMBL4551165) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526417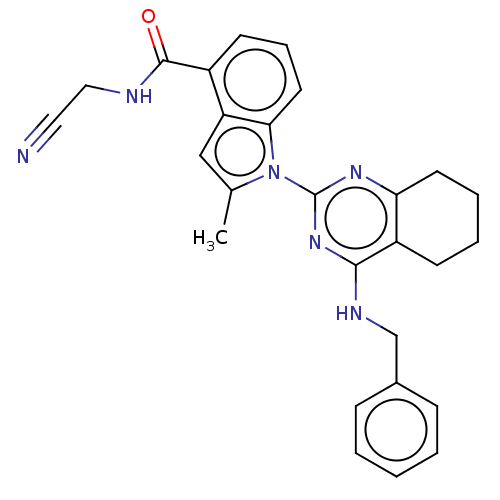 (CHEMBL4543206) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500324 (CHEMBL3747448) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526418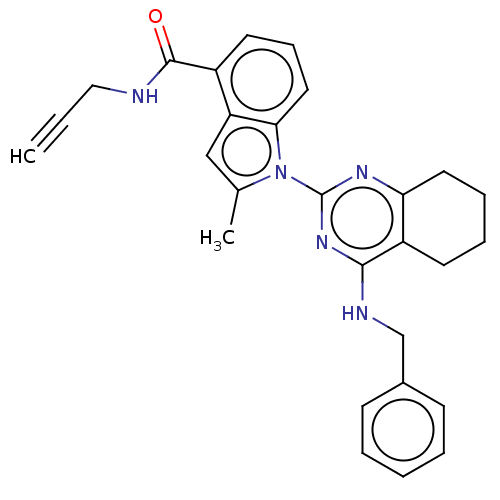 (CHEMBL4463657) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526423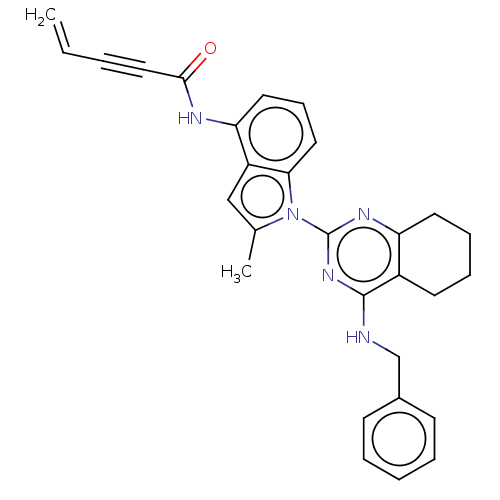 (CHEMBL4580172) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526419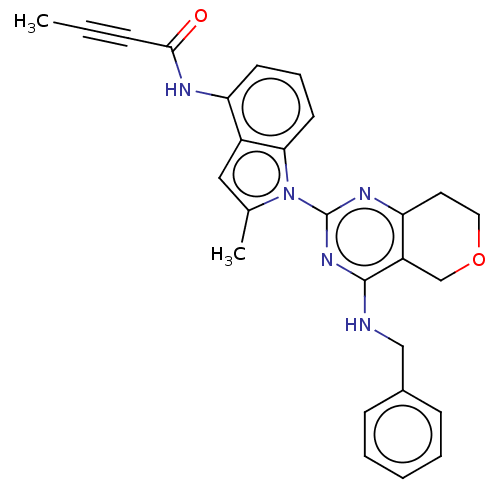 (CHEMBL4460205) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526414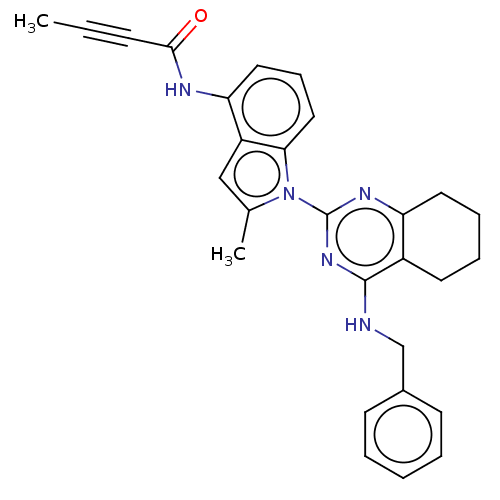 (CHEMBL4459139) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526411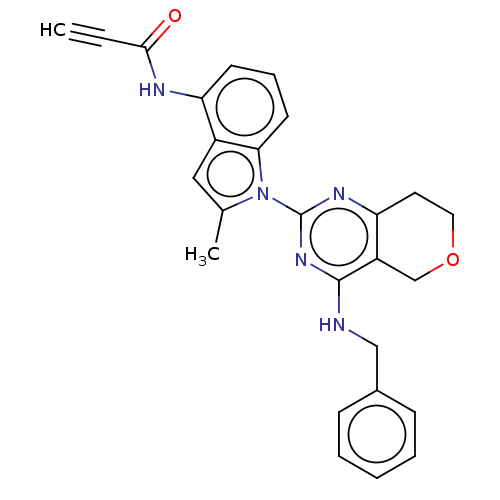 (CHEMBL4438398) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526415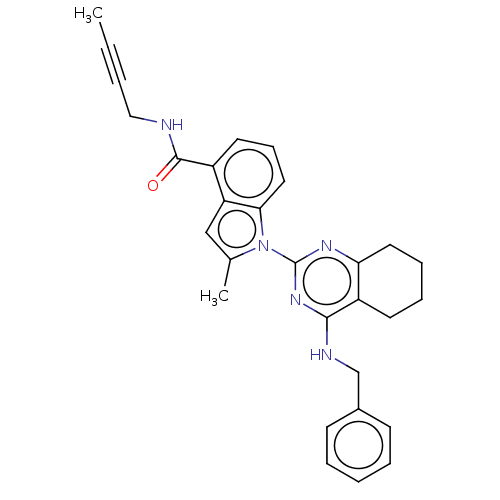 (CHEMBL4463689) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526410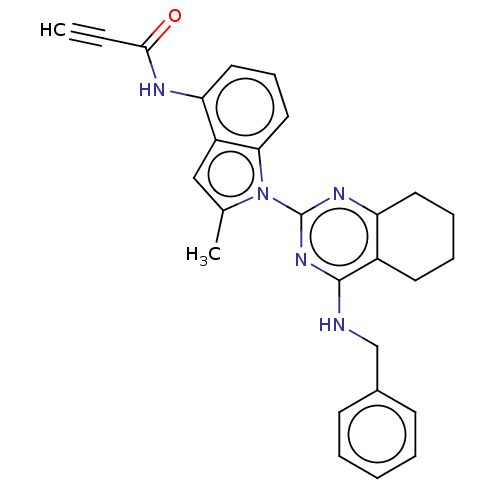 (CHEMBL4558932) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526421 (CHEMBL4446356) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526422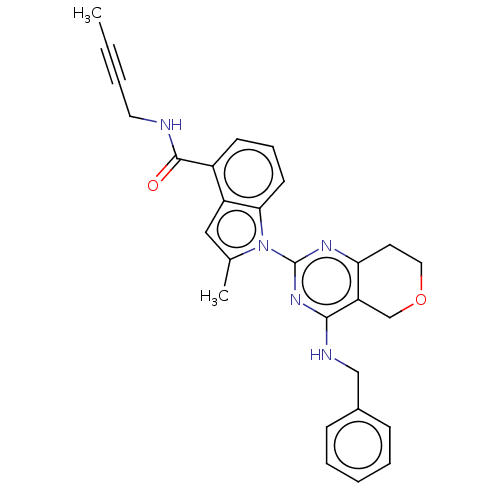 (CHEMBL4535642) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50526413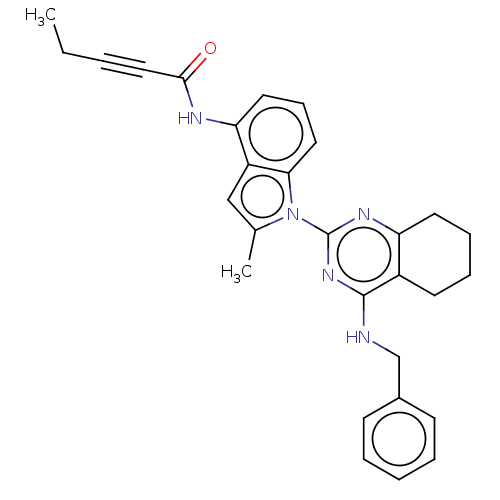 (CHEMBL4456523) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of His-tagged p97 (unknown origin) in presence of ATP and PNP by UV-transparent microplate assay | J Med Chem 62: 2814-2829 (2019) Article DOI: 10.1021/acs.jmedchem.9b00144 BindingDB Entry DOI: 10.7270/Q24171H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084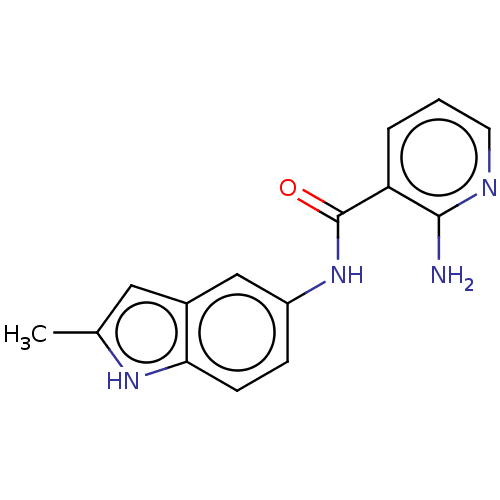 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM A... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500324 (CHEMBL3747448) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514008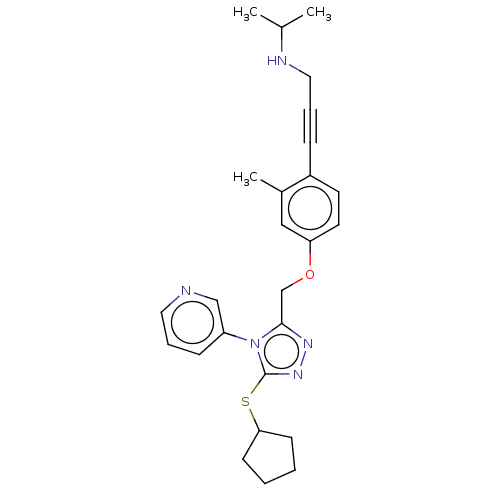 (CHEMBL4450581 | US10894782, No 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assay | J Med Chem 63: 1892-1907 (2020) Article DOI: 10.1021/acs.jmedchem.9b01318 BindingDB Entry DOI: 10.7270/Q2N58QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50587862 (CHEMBL5206855) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113446 BindingDB Entry DOI: 10.7270/Q24Q7ZXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514008 (CHEMBL4450581 | US10894782, No 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514004 (CHEMBL4476693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113446 BindingDB Entry DOI: 10.7270/Q24Q7ZXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50514004 (CHEMBL4476693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p97 (unknown origin) assessed as reduction in ATPase activity by biomol green reagent based assay | J Med Chem 63: 1892-1907 (2020) Article DOI: 10.1021/acs.jmedchem.9b01318 BindingDB Entry DOI: 10.7270/Q2N58QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478271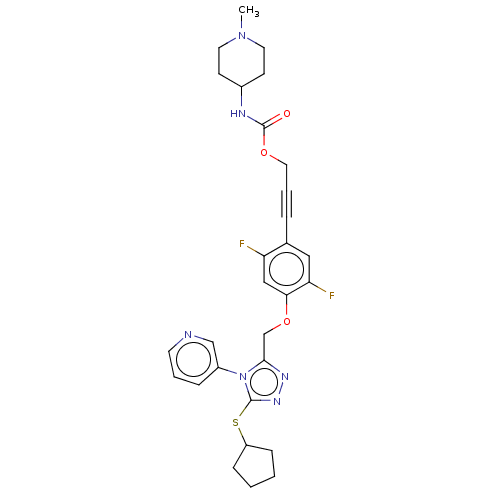 (US10894782, No 124) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478177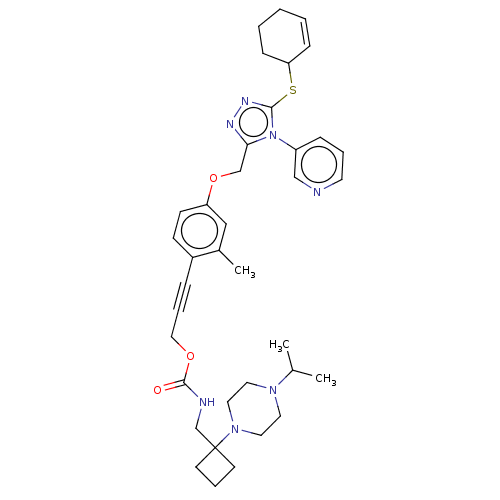 (US10894782, No 30) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50424917 (CHEMBL2311578) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 100 uM ATP as subst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500325 (Cb 5083 | Cb-5083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113446 BindingDB Entry DOI: 10.7270/Q24Q7ZXF | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500325 (Cb 5083 | Cb-5083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478267 (US10894782, No 120) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478266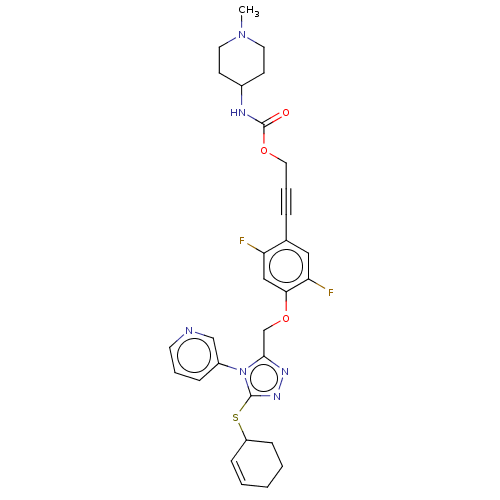 (US10894782, No 119) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478224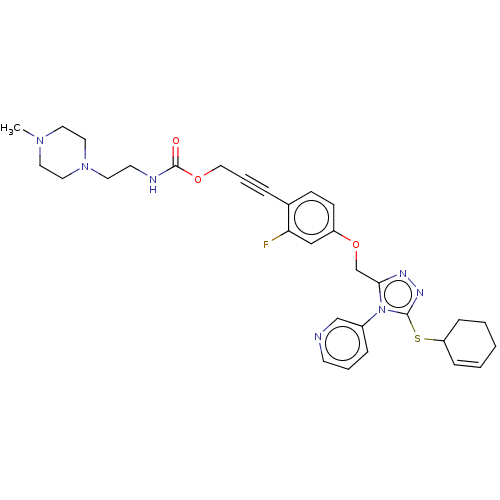 (US10894782, No 107 | US10894782, No 77) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50500319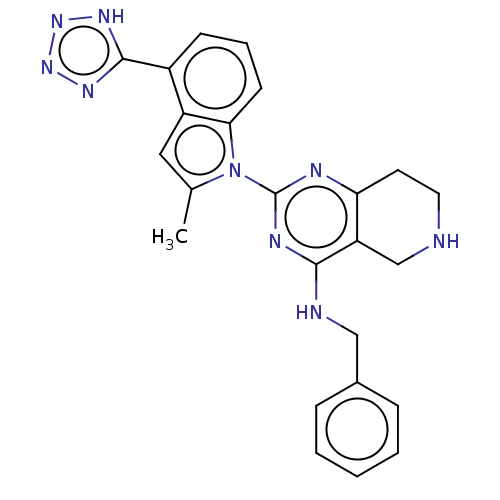 (CHEMBL3746179) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleave Biosciences Inc. Curated by ChEMBL | Assay Description Inhibition of human p97 ATPase incubated for 15 mins by ADP Glo luminescence assay | J Med Chem 58: 9480-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b01346 BindingDB Entry DOI: 10.7270/Q2KW5K2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM536747 (US11247985, Table 3.51) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... | Citation and Details BindingDB Entry DOI: 10.7270/Q25Q509B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468106 (CHEMBL4280801 | US11247985, Table 3.49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... | Citation and Details BindingDB Entry DOI: 10.7270/Q25Q509B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478222 (US10894782, No 108 | US10894782, No 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478273 (US10894782, No 126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478272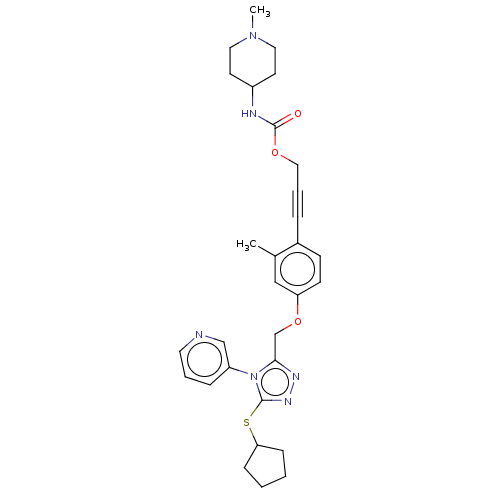 (US10894782, No 125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478265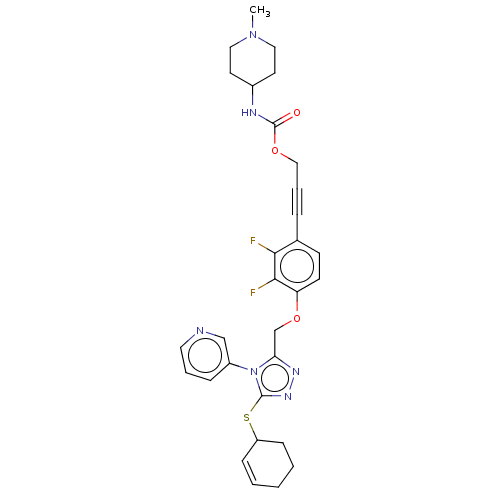 (US10894782, No 118) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478176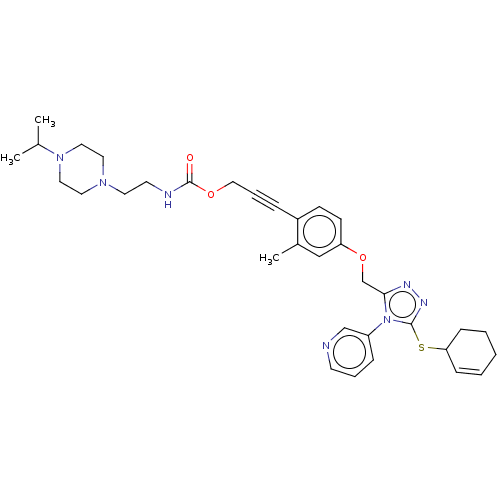 (US10894782, No 29 | US10894782, No 60 | US10894782...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478176 (US10894782, No 29 | US10894782, No 60 | US10894782...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478269 (US10894782, No 122) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468106 (CHEMBL4280801 | US11247985, Table 3.49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center Curated by ChEMBL | Assay Description Inhibition of recombinant full length human p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2 (DE3) using 100 uM ATP as substrate after... | ACS Med Chem Lett 9: 1075-1081 (2018) Article DOI: 10.1021/acsmedchemlett.8b00372 BindingDB Entry DOI: 10.7270/Q23F4SBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478224 (US10894782, No 107 | US10894782, No 77) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478261 (US10894782, No 114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468108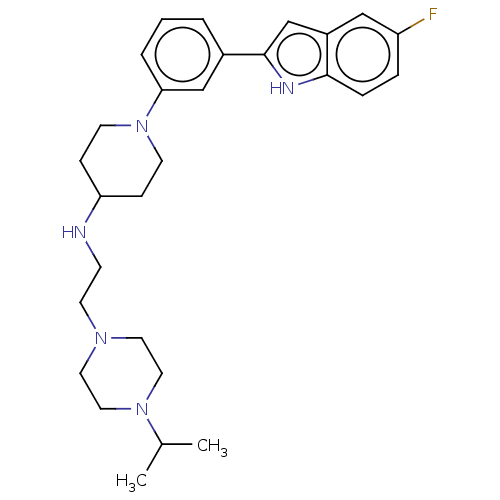 (CHEMBL4280125 | US11247985, Table 3.59) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human p97 | J Med Chem 63: 1892-1907 (2020) Article DOI: 10.1021/acs.jmedchem.9b01318 BindingDB Entry DOI: 10.7270/Q2N58QQC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM478253 (US10894782, No 106) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Biomol Green (Enzo) is a bioluminescent, homogeneous assay that measures ADP formed from a biochemical reaction. Because of its high sensitivity, the... | US Patent US10894782 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1097 total ) | Next | Last >> |