 Found 852 hits of ec50 for UniProtKB: P43490
Found 852 hits of ec50 for UniProtKB: P43490 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438936
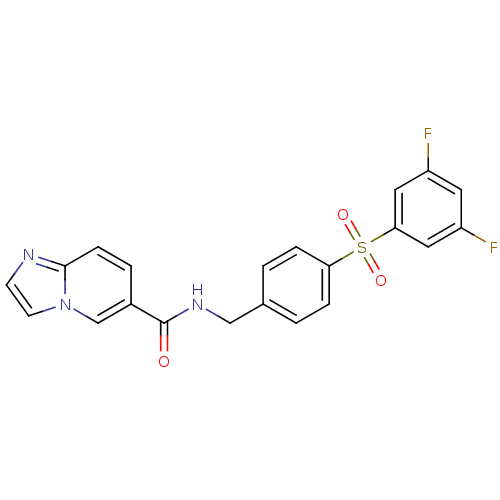
(CHEMBL2420629 | GNE-617)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)c2ccc3nccn3c2)cc1 Show InChI InChI=1S/C21H15F2N3O3S/c22-16-9-17(23)11-19(10-16)30(28,29)18-4-1-14(2-5-18)12-25-21(27)15-3-6-20-24-7-8-26(20)13-15/h1-11,13H,12H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human PC3 cells assessed as reduction in NAD level after 48 hrs by mass spectrometry |
Bioorg Med Chem Lett 23: 5488-97 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.074
BindingDB Entry DOI: 10.7270/Q2DF6SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50011266
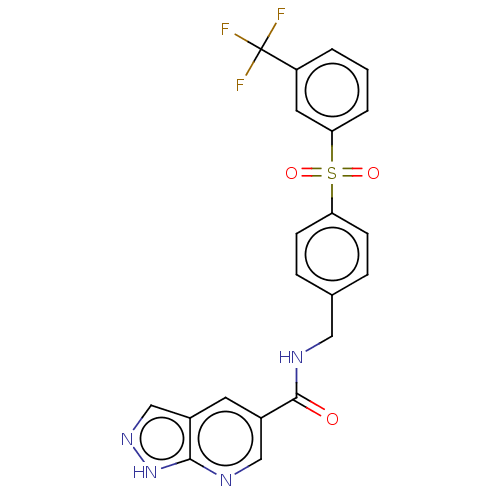
(CHEMBL3260358)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)c1ccc(CNC(=O)c2cnc3[nH]ncc3c2)cc1 Show InChI InChI=1S/C21H15F3N4O3S/c22-21(23,24)16-2-1-3-18(9-16)32(30,31)17-6-4-13(5-7-17)10-26-20(29)15-8-14-12-27-28-19(14)25-11-15/h1-9,11-12H,10H2,(H,26,29)(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human PC3 cells assessed as reduction in NAD level after 48 hrs by mass spectrometry |
Bioorg Med Chem Lett 23: 5488-97 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.074
BindingDB Entry DOI: 10.7270/Q2DF6SQ1 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50609428

(CHEMBL5266272)Show SMILES CC(NC(=O)C1CC(O)CN1C(=O)C(NC(=O)COCCOCCOCCOCCNC(=O)c1ccc(CN2CCN(CC2)S(=O)(=O)c2ccc(NC(=S)NCc3ccccc3)cc2)cc1)C(C)(C)C)c1ccc(cc1)-c1scnc1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656348
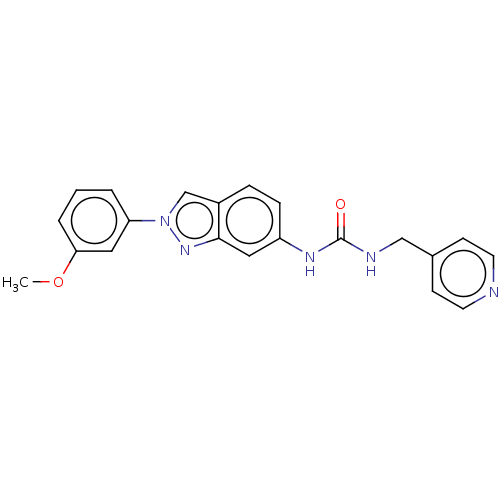
(N-[2-(3-Methoxyphenyl)-2H-indazol-6-yl]-N′-[...)Show SMILES COc1cccc(c1)-n1cc2ccc(NC(=O)NCc3ccncc3)cc2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656325
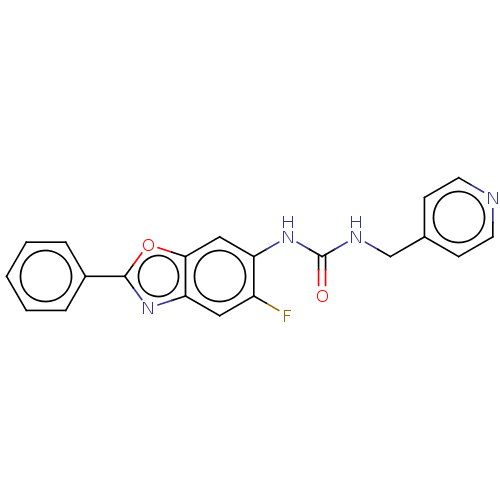
(N-(5-Fluoro-2-phenyl-1,3-benzoxazol-6-yl)-N′...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570832

(CHEMBL4848594) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656330
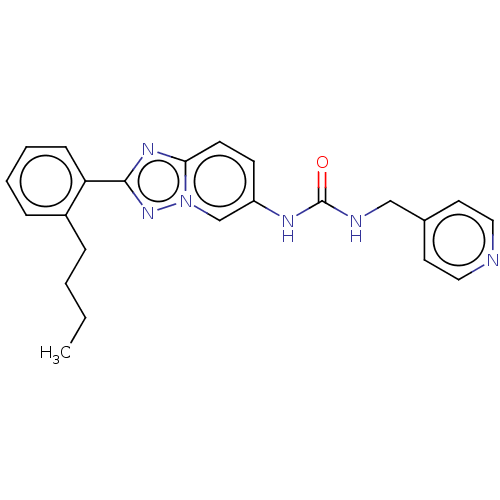
(N-[2-(2-Butylphenyl)[1,2,4]triazolo[1,5-a]pyridin-...)Show SMILES CCCCc1ccccc1-c1nc2ccc(NC(=O)NCc3ccncc3)cn2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570834
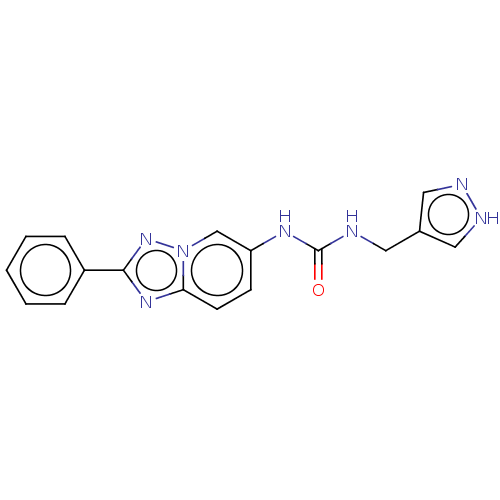
(CHEMBL4877644 | US11918568, Example 9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570834

(CHEMBL4877644 | US11918568, Example 9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572570
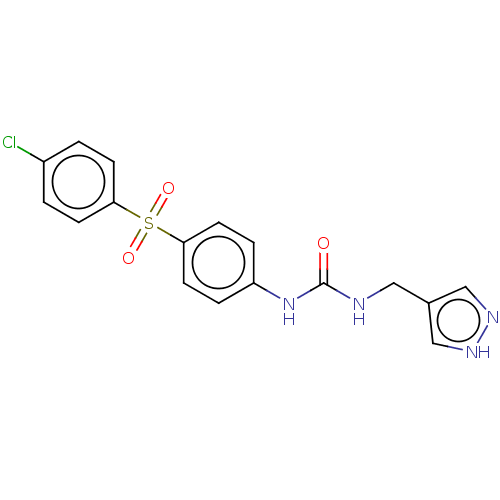
(1-[4-(4-Chloro-benzenesulfonyl)-phenyl]-3-(1H-pyra...)Show SMILES Clc1ccc(cc1)S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569533

(CHEMBL4858231 | US11452717, Compound TableB-2.166)Show SMILES CN(Cc1ccccc1)S(=O)(=O)c1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569538

(CHEMBL4859010 | US11452717, Compound TableB-1.48)Show SMILES O=C(NCc1cn[nH]c1)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656373

(N-Benzyl-N-ethyl-6-({[(pyridin-4-yl)methyl]carbamo...)Show SMILES CCN(Cc1ccccc1)C(=O)c1noc2cc(NC(=O)NCc3ccncc3)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570831
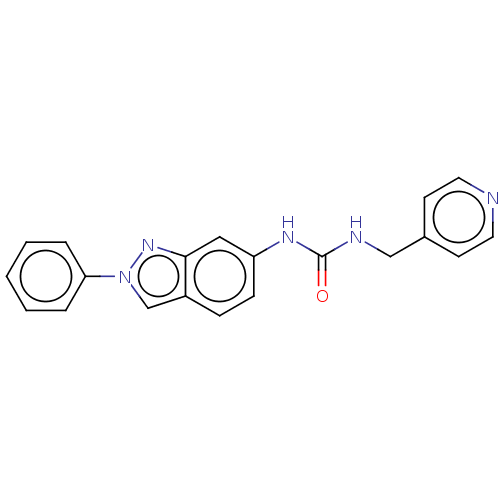
(CHEMBL4846468 | US11918568, Example 26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570831

(CHEMBL4846468 | US11918568, Example 26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656342
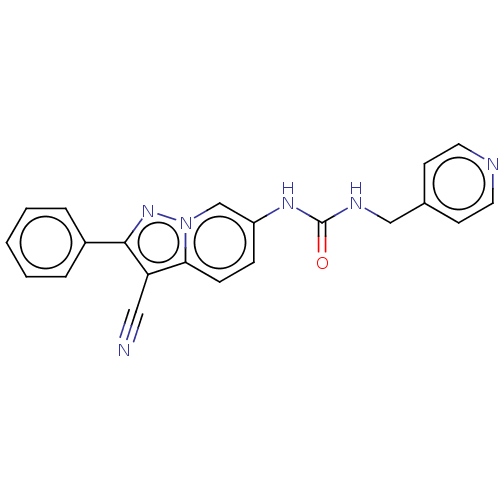
(N-(3-Cyano-2-phenylpyrazolo[1,5-a]pyridin-6-yl)-N&...)Show SMILES O=C(NCc1ccncc1)Nc1ccc2c(C#N)c(nn2c1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656336
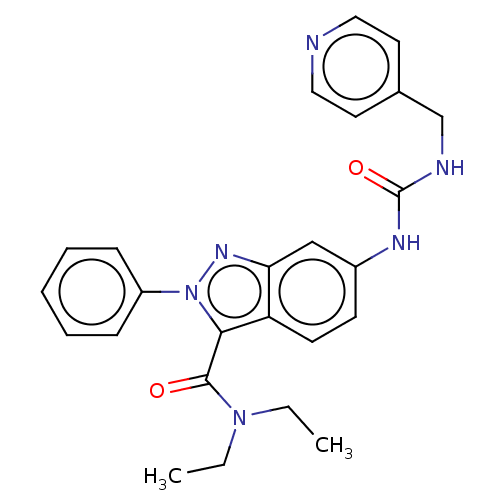
(N,N-Diethyl-2-phenyl-6-({[(pyridin-4-yl)methyl]car...)Show SMILES CCN(CC)C(=O)c1n(nc2cc(NC(=O)NCc3ccncc3)ccc12)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656321
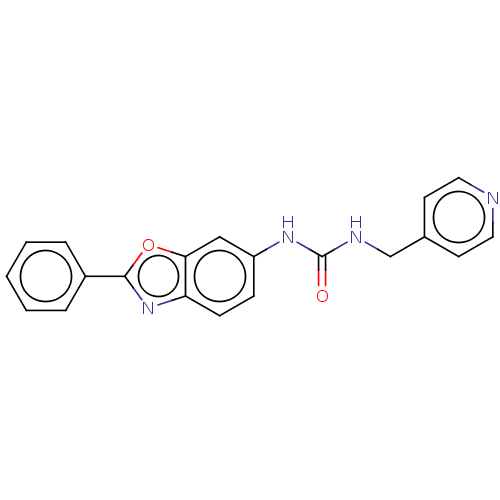
(N-(2-Phenyl-1,3-benzoxazol-6-yl)-N′-[(pyridi...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656347

(N-{2-[2-(2-Methoxyethoxy)phenyl]-2H-indazol-6-yl}-...)Show SMILES COCCOc1ccccc1-n1cc2ccc(NC(=O)NCc3ccncc3)cc2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569529
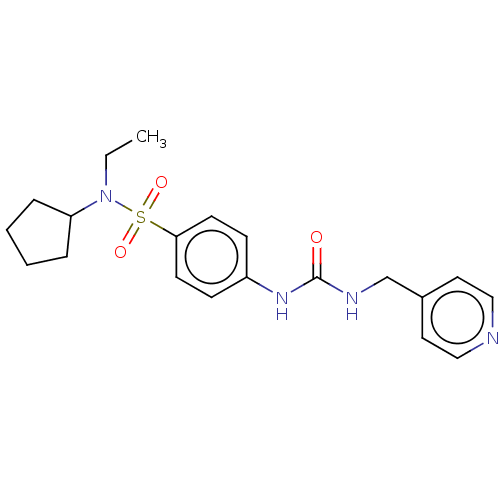
(CHEMBL4867743 | US11452717, Compound TableB-2.173)Show SMILES CCN(C1CCCC1)S(=O)(=O)c1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656363

(N-[2-(3-Methoxyphenyl)pyrazolo[1,5-a]pyridin-6-yl]...)Show SMILES COc1cccc(c1)-c1cc2ccc(NC(=O)NCc3ccncc3)cn2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569536

(CHEMBL4852773 | US11452717, Compound TableB-2.219)Show SMILES Clc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570836

(CHEMBL4860172)Show SMILES FC(F)(F)Oc1ccccc1-c1nc2ccc(NC(=O)NCc3ccncc3)cn2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656343

(N-(3-Methyl-2-phenylpyrazolo[1,5-a]pyridin-6-yl)-N...)Show SMILES Cc1c(nn2cc(NC(=O)NCc3ccncc3)ccc12)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569541

(CHEMBL4859855 | US11452717, Compound TableB-5.29)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)NCc2cnco2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569540
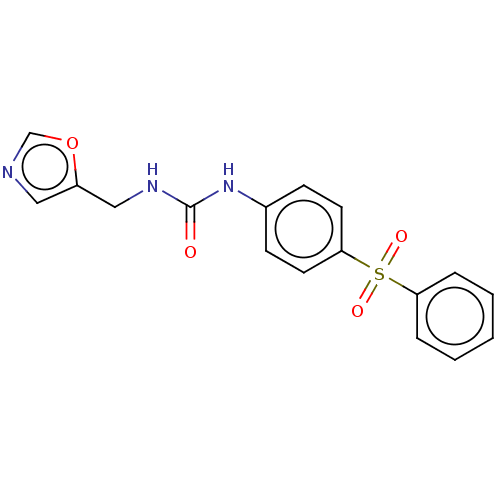
(CHEMBL4868976 | US11452717, Compound TableB-1.47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569535

(CHEMBL4856434 | US11452717, Compound TableB-2.221)Show SMILES Clc1ccccc1S(=O)(=O)c1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656340

(N-[3-(1-Methyl-1H-pyrazol-5-yl)-2-phenylpyrazolo[1...)Show SMILES Cn1nccc1-c1c(nn2cc(NC(=O)NCc3ccncc3)ccc12)-c1ccccc1 |(2,3.28,;3.54,3.28,;4.45,4.53,;5.91,4.05,;5.91,2.51,;4.45,2.03,;3.97,.57,;4.88,-.68,;3.97,-1.92,;2.51,-1.45,;1.17,-2.22,;-.16,-1.45,;-1.49,-2.22,;-2.83,-1.45,;-2.83,.09,;-4.16,-2.22,;-5.5,-1.45,;-6.83,-2.22,;-6.83,-3.76,;-8.16,-4.53,;-9.5,-3.76,;-9.5,-2.22,;-8.16,-1.45,;-.16,.09,;1.17,.86,;2.51,.09,;6.42,-.68,;7.19,.66,;8.73,.66,;9.5,-.68,;8.73,-2.01,;7.19,-2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570833
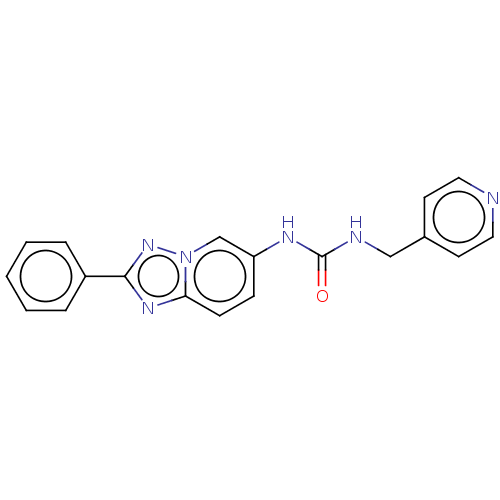
(CHEMBL4861097 | US11918568, Example 8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656370
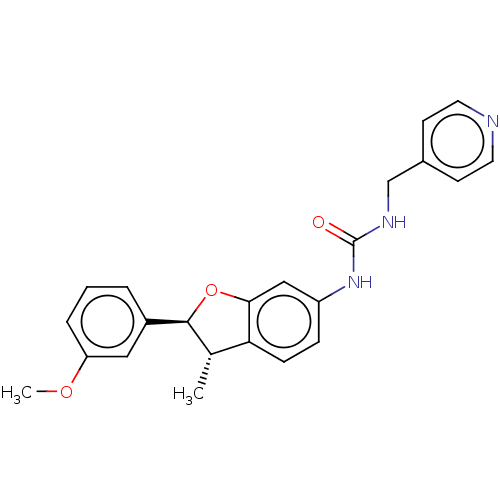
(N-[(2S*,3S*)-2-(3-Methoxyphenyl)-3-methyl-2,3-dihy...)Show SMILES COc1cccc(c1)[C@H]1Oc2cc(NC(=O)NCc3ccncc3)ccc2[C@@H]1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656369

(N-[2-(3-Methoxyphenyl)-2,3-dihydro-1-benzofuran-6-...)Show SMILES COc1cccc(c1)C1Cc2ccc(NC(=O)NCc3ccncc3)cc2O1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570833

(CHEMBL4861097 | US11918568, Example 8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50570844
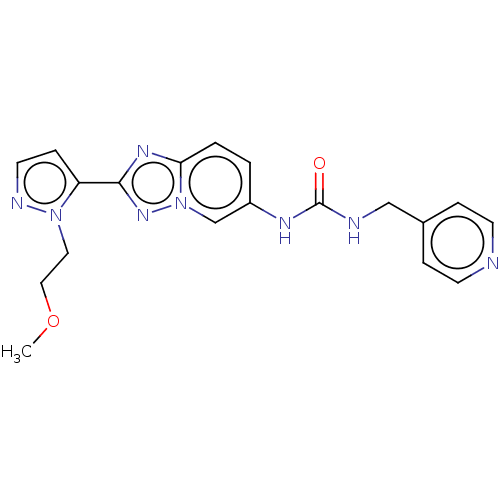
(CHEMBL4877360)Show SMILES COCCn1nccc1-c1nc2ccc(NC(=O)NCc3ccncc3)cn2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128048
BindingDB Entry DOI: 10.7270/Q27W6H07 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50569534
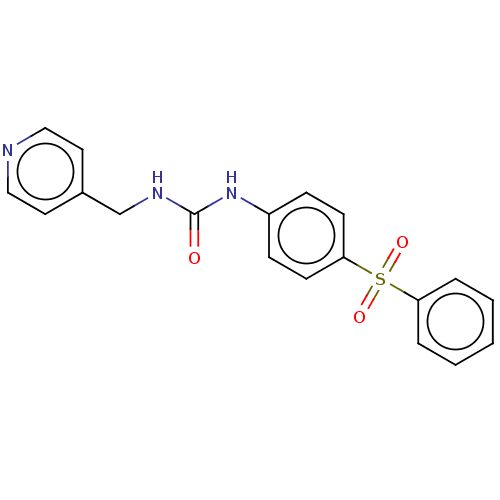
(CHEMBL4868692 | US11452717, Compound TableB-2.224)Show SMILES O=C(NCc1ccncc1)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of human NAMPT |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128007
BindingDB Entry DOI: 10.7270/Q2MK6HPH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM656360
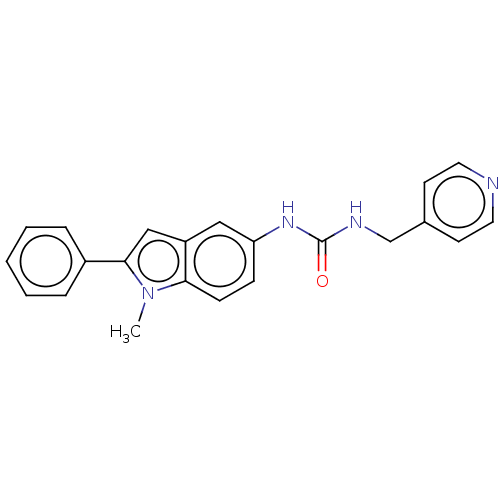
(N-(1-Methyl-2-phenyl-1H-indol-5-yl)-N′-[(pyr...)Show SMILES Cn1c(cc2cc(NC(=O)NCc3ccncc3)ccc12)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572812
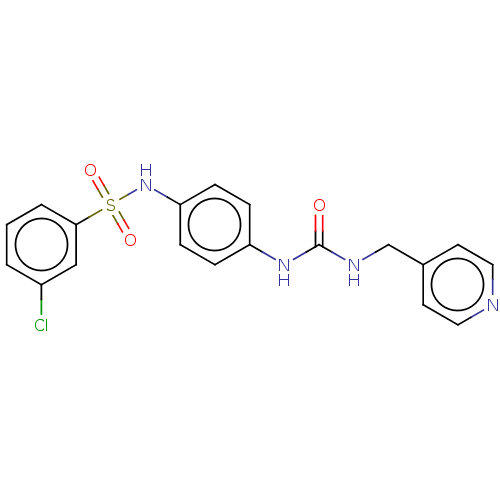
(3-Chloro-N-[4-(3-pyridin-4-ylmethyl-ureido)-phenyl...)Show SMILES Clc1cccc(c1)S(=O)(=O)Nc1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572813
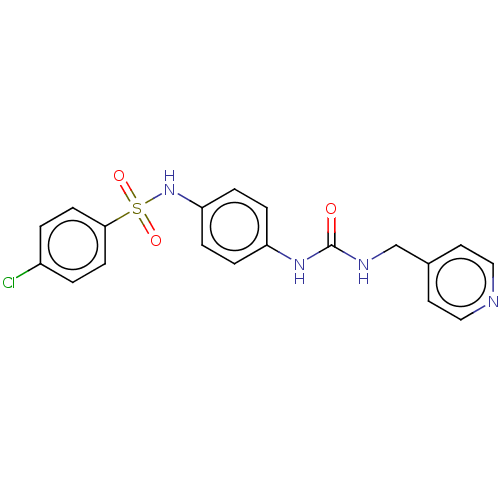
(4-Chloro-N-[4-(3-pyridin-4-ylmethyl-ureido)-phenyl...)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572814

(N-[4-(3-Benzyl-ureido)-phenyl]-C-phenyl-methanesul...)Show SMILES O=C(NCc1ccccc1)Nc1ccc(NS(=O)(=O)Cc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572815

(1-(2-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...)Show SMILES Clc1ccccc1CS(=O)(=O)Nc1ccc(NC(=O)NCc2ccncc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572816

(1-(3-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...)Show SMILES Clc1cccc(CS(=O)(=O)Nc2ccc(NC(=O)NCc3ccncc3)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572817
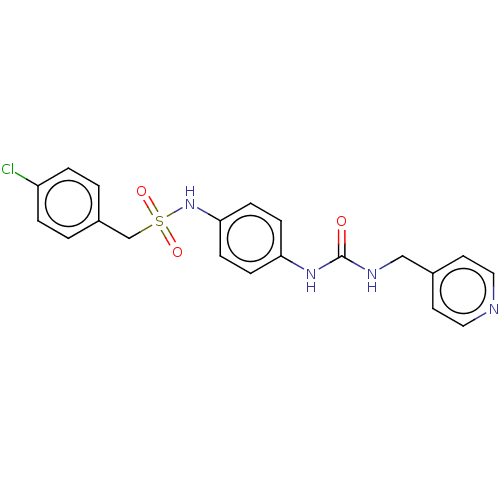
(1-(4-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...)Show SMILES Clc1ccc(CS(=O)(=O)Nc2ccc(NC(=O)NCc3ccncc3)cc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572818
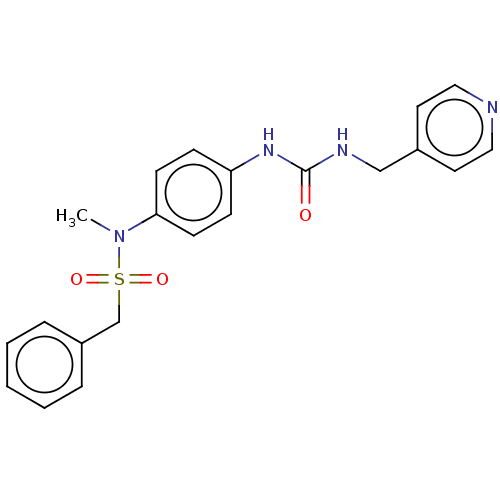
(N-Methyl-C-phenyl-N-[4-(3-pyridin-4-ylmethyl-ureid...)Show SMILES CN(c1ccc(NC(=O)NCc2ccncc2)cc1)S(=O)(=O)Cc1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572861
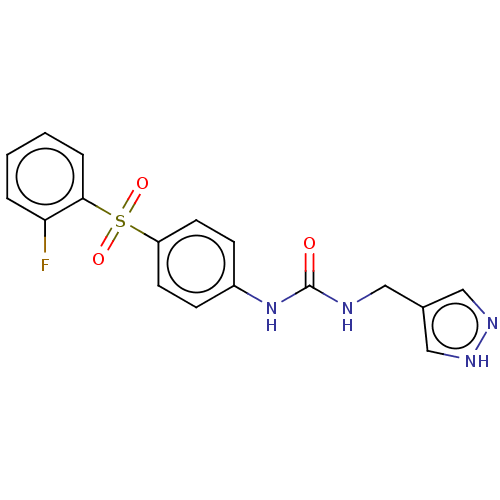
(1-((1H-Pyrazol-4-yl)methyl)-3-(4-((2-fluorophenyl)...)Show SMILES Fc1ccccc1S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572862
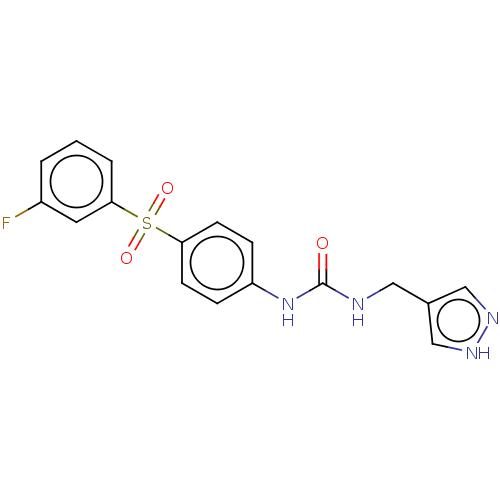
(1-((1H-Pyrazol-4-yl)methyl)-3-(4-((3-fluorophenyl)...)Show SMILES Fc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572863

(1-((1H-Pyrazol-4-yl)methyl)-3-(4-((4-fluorophenyl)...)Show SMILES Fc1ccc(cc1)S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572864

(1-((1H-Pyrazol-4-yl)methyl)-3-(4-((3-(trifluoromet...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572869
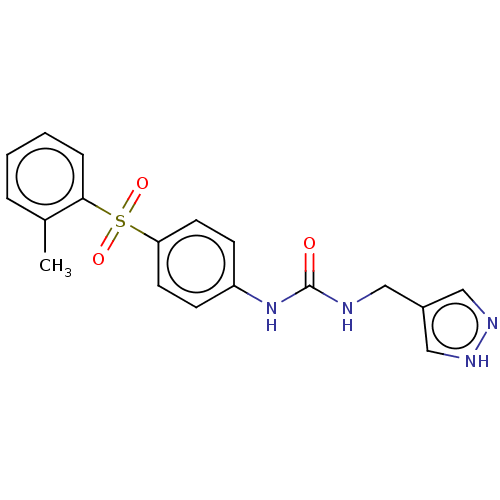
(1-(1H-Pyrazol-4-ylmethyl)-3-[4-(toluene-2-sulfonyl...)Show SMILES Cc1ccccc1S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572871

(1-[4-(3,4-Dichloro-benzenesulfonyl)-phenyl]-3-(1H-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)c1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572872

(C-(3-Chloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylmethyl...)Show SMILES Clc1cccc(CS(=O)(=O)Nc2ccc(NC(=O)NCc3cn[nH]c3)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM572873
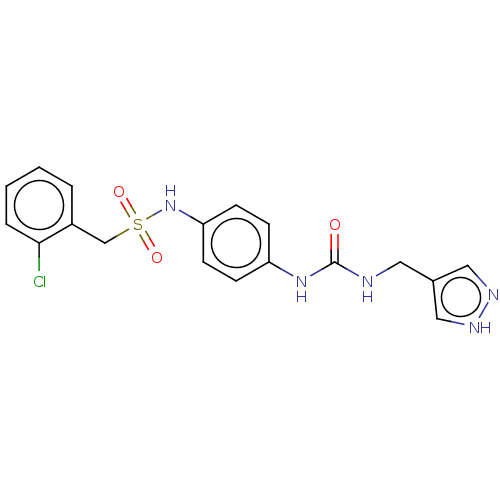
(C-(2-Chloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylmethyl...)Show SMILES Clc1ccccc1CS(=O)(=O)Nc1ccc(NC(=O)NCc2cn[nH]c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M048P0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data