

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298425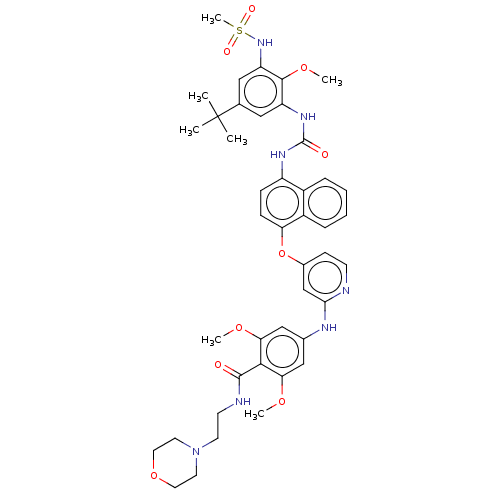 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298425 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298425 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298425 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||