

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393389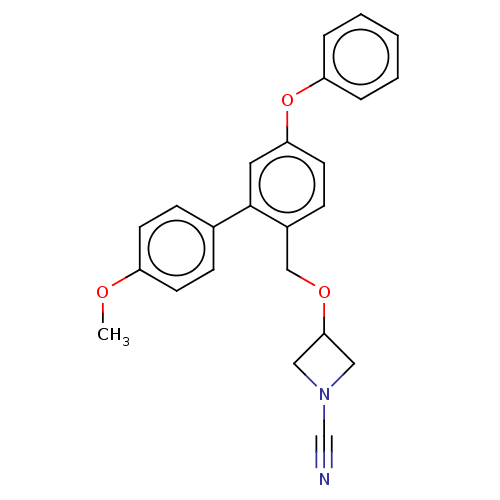 (3-((4'-Methoxy-5-phenoxy-[1,1'-biphenyl]-2-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393389 (3-((4'-Methoxy-5-phenoxy-[1,1'-biphenyl]-2-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,... | Chem Biol Drug Des 71: 131-9 (2008) BindingDB Entry DOI: 10.7270/Q2639S2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||