

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM409572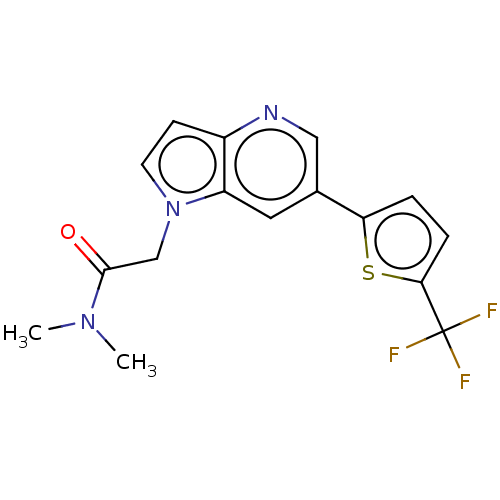 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant human GluN1a/GluN2B expressed in CHO-T-REx cells assessed as inhibition of glutamate/glycine-induced re... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO cells measured after 5 mins by Qpatch method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02113 BindingDB Entry DOI: 10.7270/Q2V128JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from recombinant human ERG expressed in HEK293 cell membranes measured after 80 mins by TopCount scintillation analysi... | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM409572 (N,N-Dimethyl-2-[6-[5-(trifluoromethyl)-2-thienyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | ACS Med Chem Lett 10: 261-266 (2019) Article DOI: 10.1021/acsmedchemlett.8b00542 BindingDB Entry DOI: 10.7270/Q2B85CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||