

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034509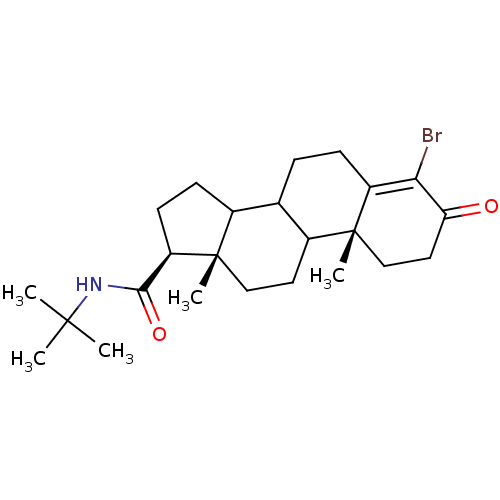 ((10R,13S,17S)-4-Bromo-10,13-dimethyl-3-oxo-2,3,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Inhibitory concentration on human 5-alpha reductase 2 (transfected SW-13 cells) | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50034509 ((10R,13S,17S)-4-Bromo-10,13-dimethyl-3-oxo-2,3,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human Steroid 5-alpha-reductase type 2 in transfected SW-13 cells using [3H]- delta4-Androstenedione as substrate | J Med Chem 38: 1456-61 (1995) BindingDB Entry DOI: 10.7270/Q2W096K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034509 ((10R,13S,17S)-4-Bromo-10,13-dimethyl-3-oxo-2,3,6,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 981 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Inhibitory concentration on human 5-alpha reductase 1 (transfected 293 cells) | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50034509 ((10R,13S,17S)-4-Bromo-10,13-dimethyl-3-oxo-2,3,6,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 981 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center Curated by ChEMBL | Assay Description In vitro inhibition of human tSteroid 5-alpha-reductase type I in transfected 293 cells using [3H]- delta4-Androstenedione as substrate. | J Med Chem 38: 1456-61 (1995) BindingDB Entry DOI: 10.7270/Q2W096K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||