

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50148173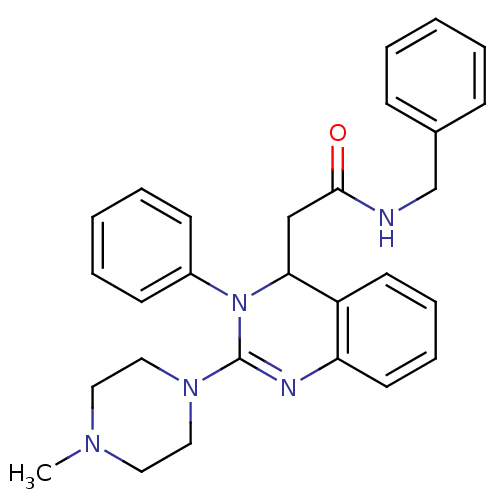 (CHEMBL326854 | N-Benzyl-2-[2-(4-methyl-piperazin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 5 m... | Bioorg Med Chem Lett 27: 1179-1185 (2017) Article DOI: 10.1016/j.bmcl.2017.01.068 BindingDB Entry DOI: 10.7270/Q2P271CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50148173 (CHEMBL326854 | N-Benzyl-2-[2-(4-methyl-piperazin-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 5 mi... | Bioorg Med Chem Lett 27: 1179-1185 (2017) Article DOI: 10.1016/j.bmcl.2017.01.068 BindingDB Entry DOI: 10.7270/Q2P271CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50148173 (CHEMBL326854 | N-Benzyl-2-[2-(4-methyl-piperazin-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Concentration of the compounds required for inhibition of HEK293 cells (alpha1G T-type) | Bioorg Med Chem Lett 14: 3379-84 (2004) Article DOI: 10.1016/j.bmcl.2004.04.090 BindingDB Entry DOI: 10.7270/Q21Z43VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50148173 (CHEMBL326854 | N-Benzyl-2-[2-(4-methyl-piperazin-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human T-type calcium channel alpha1G in HEK293 cells | Bioorg Med Chem Lett 20: 38-41 (2010) Article DOI: 10.1016/j.bmcl.2009.11.049 BindingDB Entry DOI: 10.7270/Q2MS3V0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||