 Found 5 hits of ic50 for monomerid = 50192797
Found 5 hits of ic50 for monomerid = 50192797 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50192797
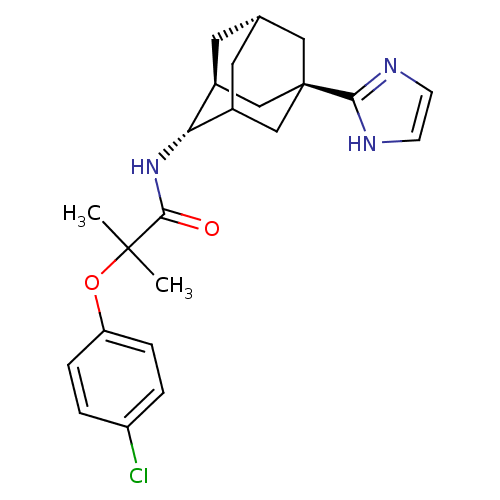
(2-(4-chloro-phenoxy)-N-[(1R,2S,5S,7S)-5-(1H-imidaz...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@H]1C[C@](C3)(C2)c1ncc[nH]1 |wU:17.24,14.14,wD:19.19,21.27,TLB:13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,(3.46,-5.22,;2.14,-6,;.81,-5.23,;3.47,-6.79,;4.78,-5.98,;6.13,-6.71,;7.43,-5.9,;7.38,-4.36,;8.69,-3.54,;6.02,-3.63,;4.72,-4.45,;.79,-6.76,;.78,-8.3,;-.53,-5.97,;-1.81,-6.82,;-1.83,-8.35,;-2.84,-9.63,;-4.25,-9.07,;-4.25,-7.48,;-3.21,-6.24,;-4.56,-6.72,;-4.55,-8.21,;-5.75,-9.49,;-3.22,-8.7,;-5.89,-7.43,;-6.05,-5.9,;-7.57,-5.57,;-8.34,-6.91,;-7.3,-8.06,)| Show InChI InChI=1S/C23H28ClN3O2/c1-22(2,29-18-5-3-17(24)4-6-18)21(28)27-19-15-9-14-10-16(19)13-23(11-14,12-15)20-25-7-8-26-20/h3-8,14-16,19H,9-13H2,1-2H3,(H,25,26)(H,27,28)/t14-,15-,16?,19+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli |
Bioorg Med Chem Lett 16: 5414-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.055
BindingDB Entry DOI: 10.7270/Q28S4PJ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50192797

(2-(4-chloro-phenoxy)-N-[(1R,2S,5S,7S)-5-(1H-imidaz...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@H]1C[C@](C3)(C2)c1ncc[nH]1 |wU:17.24,14.14,wD:19.19,21.27,TLB:13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,(3.46,-5.22,;2.14,-6,;.81,-5.23,;3.47,-6.79,;4.78,-5.98,;6.13,-6.71,;7.43,-5.9,;7.38,-4.36,;8.69,-3.54,;6.02,-3.63,;4.72,-4.45,;.79,-6.76,;.78,-8.3,;-.53,-5.97,;-1.81,-6.82,;-1.83,-8.35,;-2.84,-9.63,;-4.25,-9.07,;-4.25,-7.48,;-3.21,-6.24,;-4.56,-6.72,;-4.55,-8.21,;-5.75,-9.49,;-3.22,-8.7,;-5.89,-7.43,;-6.05,-5.9,;-7.57,-5.57,;-8.34,-6.91,;-7.3,-8.06,)| Show InChI InChI=1S/C23H28ClN3O2/c1-22(2,29-18-5-3-17(24)4-6-18)21(28)27-19-15-9-14-10-16(19)13-23(11-14,12-15)20-25-7-8-26-20/h3-8,14-16,19H,9-13H2,1-2H3,(H,25,26)(H,27,28)/t14-,15-,16?,19+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in Escherichia coli |
Bioorg Med Chem Lett 16: 5414-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.055
BindingDB Entry DOI: 10.7270/Q28S4PJ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50192797

(2-(4-chloro-phenoxy)-N-[(1R,2S,5S,7S)-5-(1H-imidaz...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@H]1C[C@](C3)(C2)c1ncc[nH]1 |wU:17.24,14.14,wD:19.19,21.27,TLB:13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,(3.46,-5.22,;2.14,-6,;.81,-5.23,;3.47,-6.79,;4.78,-5.98,;6.13,-6.71,;7.43,-5.9,;7.38,-4.36,;8.69,-3.54,;6.02,-3.63,;4.72,-4.45,;.79,-6.76,;.78,-8.3,;-.53,-5.97,;-1.81,-6.82,;-1.83,-8.35,;-2.84,-9.63,;-4.25,-9.07,;-4.25,-7.48,;-3.21,-6.24,;-4.56,-6.72,;-4.55,-8.21,;-5.75,-9.49,;-3.22,-8.7,;-5.89,-7.43,;-6.05,-5.9,;-7.57,-5.57,;-8.34,-6.91,;-7.3,-8.06,)| Show InChI InChI=1S/C23H28ClN3O2/c1-22(2,29-18-5-3-17(24)4-6-18)21(28)27-19-15-9-14-10-16(19)13-23(11-14,12-15)20-25-7-8-26-20/h3-8,14-16,19H,9-13H2,1-2H3,(H,25,26)(H,27,28)/t14-,15-,16?,19+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5414-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.055
BindingDB Entry DOI: 10.7270/Q28S4PJ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Mus musculus (mouse)) | BDBM50192797

(2-(4-chloro-phenoxy)-N-[(1R,2S,5S,7S)-5-(1H-imidaz...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@H]1C[C@](C3)(C2)c1ncc[nH]1 |wU:17.24,14.14,wD:19.19,21.27,TLB:13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,(3.46,-5.22,;2.14,-6,;.81,-5.23,;3.47,-6.79,;4.78,-5.98,;6.13,-6.71,;7.43,-5.9,;7.38,-4.36,;8.69,-3.54,;6.02,-3.63,;4.72,-4.45,;.79,-6.76,;.78,-8.3,;-.53,-5.97,;-1.81,-6.82,;-1.83,-8.35,;-2.84,-9.63,;-4.25,-9.07,;-4.25,-7.48,;-3.21,-6.24,;-4.56,-6.72,;-4.55,-8.21,;-5.75,-9.49,;-3.22,-8.7,;-5.89,-7.43,;-6.05,-5.9,;-7.57,-5.57,;-8.34,-6.91,;-7.3,-8.06,)| Show InChI InChI=1S/C23H28ClN3O2/c1-22(2,29-18-5-3-17(24)4-6-18)21(28)27-19-15-9-14-10-16(19)13-23(11-14,12-15)20-25-7-8-26-20/h3-8,14-16,19H,9-13H2,1-2H3,(H,25,26)(H,27,28)/t14-,15-,16?,19+,23+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD2 |
Bioorg Med Chem Lett 16: 5414-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.055
BindingDB Entry DOI: 10.7270/Q28S4PJ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50192797

(2-(4-chloro-phenoxy)-N-[(1R,2S,5S,7S)-5-(1H-imidaz...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2C[C@@H]3C[C@H]1C[C@](C3)(C2)c1ncc[nH]1 |wU:17.24,14.14,wD:19.19,21.27,TLB:13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,(3.46,-5.22,;2.14,-6,;.81,-5.23,;3.47,-6.79,;4.78,-5.98,;6.13,-6.71,;7.43,-5.9,;7.38,-4.36,;8.69,-3.54,;6.02,-3.63,;4.72,-4.45,;.79,-6.76,;.78,-8.3,;-.53,-5.97,;-1.81,-6.82,;-1.83,-8.35,;-2.84,-9.63,;-4.25,-9.07,;-4.25,-7.48,;-3.21,-6.24,;-4.56,-6.72,;-4.55,-8.21,;-5.75,-9.49,;-3.22,-8.7,;-5.89,-7.43,;-6.05,-5.9,;-7.57,-5.57,;-8.34,-6.91,;-7.3,-8.06,)| Show InChI InChI=1S/C23H28ClN3O2/c1-22(2,29-18-5-3-17(24)4-6-18)21(28)27-19-15-9-14-10-16(19)13-23(11-14,12-15)20-25-7-8-26-20/h3-8,14-16,19H,9-13H2,1-2H3,(H,25,26)(H,27,28)/t14-,15-,16?,19+,23+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD2 |
Bioorg Med Chem Lett 16: 5414-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.055
BindingDB Entry DOI: 10.7270/Q28S4PJ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data