

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50241166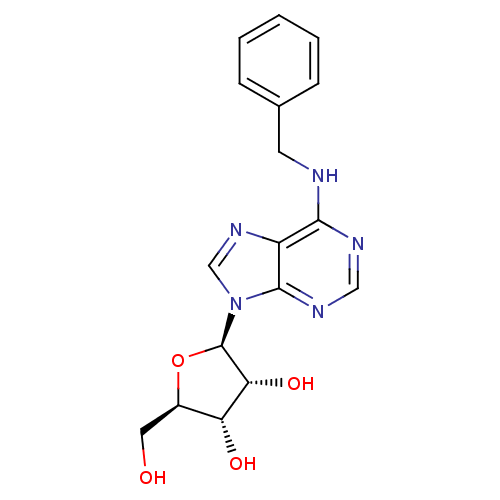 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Evaluated for binding affinity against Adenosine A1 receptor | J Med Chem 35: 629-35 (1992) Checked by Author BindingDB Entry DOI: 10.7270/Q23R0V5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50241166 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity to ENT1 transporter | Bioorg Med Chem 16: 3848-65 (2008) Article DOI: 10.1016/j.bmc.2008.01.044 BindingDB Entry DOI: 10.7270/Q2GM8866 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50241166 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 7.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Toxoplasma gondii adenosine kinase | Citation and Details Article DOI: 10.1007/s00044-011-9554-z BindingDB Entry DOI: 10.7270/Q29G5QQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoglycerate kinase 1 (Homo sapiens (Human)) | BDBM50241166 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description evaluated for the inhibition of Trypanosoma brucei Phosphoglycerate kinase (PGK) | J Med Chem 43: 4135-50 (2000) Article DOI: 10.1021/jm000287a BindingDB Entry DOI: 10.7270/Q2TB19N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50241166 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibitory activity measured for Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Leishmania. mexicana | J Med Chem 41: 4790-9 (1998) Article DOI: 10.1021/jm9802620 BindingDB Entry DOI: 10.7270/Q2Q240ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||