

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50302925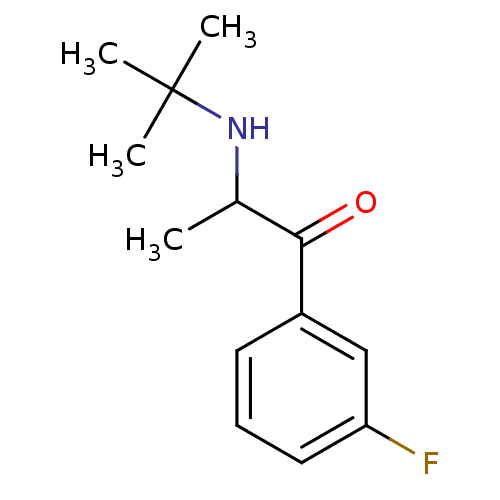 (2-(tert-Butylamino)-3'-fluoropropiophenone | 2-(te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... | J Med Chem 53: 2204-14 (2010) Article DOI: 10.1021/jm9017465 BindingDB Entry DOI: 10.7270/Q2FN1693 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50302925 (2-(tert-Butylamino)-3'-fluoropropiophenone | 2-(te...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin uptake at human SERT expressed in HEK293 cells | J Med Chem 53: 2204-14 (2010) Article DOI: 10.1021/jm9017465 BindingDB Entry DOI: 10.7270/Q2FN1693 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50302925 (2-(tert-Butylamino)-3'-fluoropropiophenone | 2-(te...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine reuptake at human DAT expressed in HEK293 cells | J Med Chem 52: 6768-81 (2009) Checked by Author Article DOI: 10.1021/jm901189z BindingDB Entry DOI: 10.7270/Q2ZK5HMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50302925 (2-(tert-Butylamino)-3'-fluoropropiophenone | 2-(te...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine uptake at human DAT expressed in HEK293 cells | J Med Chem 53: 2204-14 (2010) Article DOI: 10.1021/jm9017465 BindingDB Entry DOI: 10.7270/Q2FN1693 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50302925 (2-(tert-Butylamino)-3'-fluoropropiophenone | 2-(te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]norepinephrine uptake at human NET expressed in HEK293 cells | J Med Chem 53: 2204-14 (2010) Article DOI: 10.1021/jm9017465 BindingDB Entry DOI: 10.7270/Q2FN1693 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||