

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Putative glycogen synthase kinase 3 alpha (Ustilago maydis (Smut fungus)) | BDBM50362103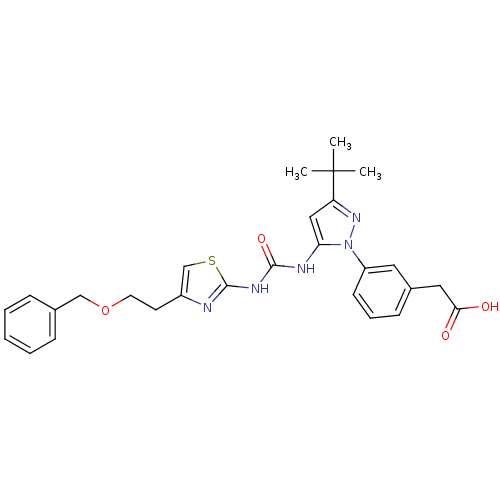 (CHEMBL1940503 | Thiazole-urea, 10) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Dortmund | Assay Description Inhibition of UmGSK3 by kinase inhibitors. | ACS Chem Biol 7: 1257-67 (2012) Article DOI: 10.1021/cb300128b BindingDB Entry DOI: 10.7270/Q29S1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50362103 (CHEMBL1940503 | Thiazole-urea, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of wild type DDR2 (unknown origin) preincubated for 30 mins before substrate addition by FRET assay | J Med Chem 57: 4252-62 (2014) Article DOI: 10.1021/jm500167q BindingDB Entry DOI: 10.7270/Q2Z039P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||