

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468123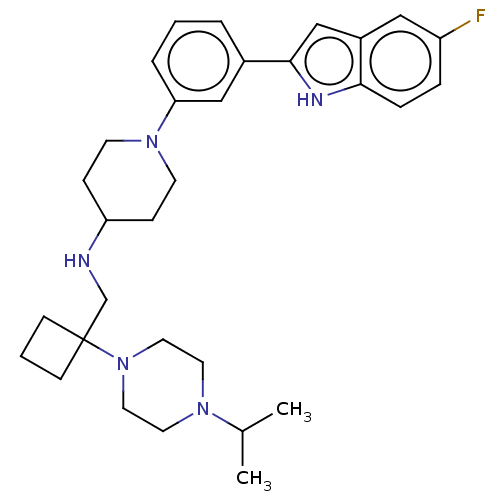 (CHEMBL4291440 | US11247985, Table 3.97) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center Curated by ChEMBL | Assay Description Inhibition of recombinant full length human p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2 (DE3) using 100 uM ATP as substrate after... | ACS Med Chem Lett 9: 1075-1081 (2018) Article DOI: 10.1021/acsmedchemlett.8b00372 BindingDB Entry DOI: 10.7270/Q23F4SBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50468123 (CHEMBL4291440 | US11247985, Table 3.97) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... | Citation and Details BindingDB Entry DOI: 10.7270/Q25Q509B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||