

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539946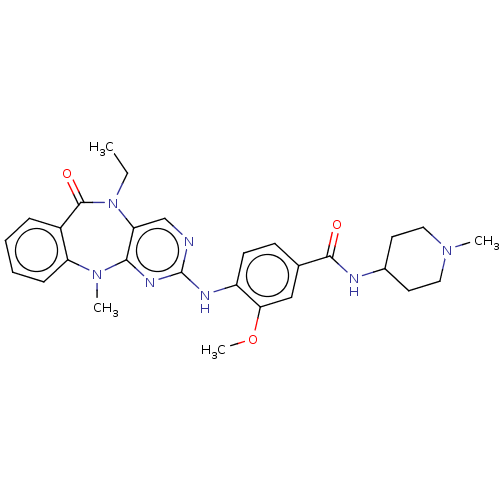 (CHEMBL4643632) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50539946 (CHEMBL4643632) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 7 (Homo sapiens (Human)) | BDBM50539946 (CHEMBL4643632) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of ERK5 in human HeLa cells assessed as reduction in EGF-induced ERK5 autophosphorylation pretreated for 1 hr followed by EGF stimulation ... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50539946 (CHEMBL4643632) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of BRD4 bromodomain 1 (unknown origin) by AlphaScreen displacement assay | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||